Method for sorting human marrow mesenchymal stem cell by monoclonal antibody ZUE12 immunomagnetic bead
A technology of immunomagnetic bead sorting and ZUE12, which is applied in the direction of bone/connective tissue cells, animal cells, vertebrate cells, etc., can solve the problem of complex components of CD45-GlycophorinA- cell populations, and it is difficult to directly sort human bone marrow MSCs by immunolabeling Problems such as ideal labeling, MSCs separation, and practical application of purification are difficult to achieve the effects of improving time-consuming, shortening time, and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Separation of primary human bone marrow MSCs by immunomagnetic bead method with monoclonal antibody ZUE12
[0023] Bone marrow was collected from a healthy donor, heparin anticoagulated, and Ficoll-paque (specific gravity 1.077) density gradient centrifugation was used to obtain bone marrow mononuclear cells. The nuclear cells were incubated, centrifuged and washed, then incubated with anti-mouse IgG magnetic bead secondary antibody (Miltenyi Biotec Inc.), centrifuged and washed, and isolated from bone marrow mononuclear cells by immunomagnetic bead sorting system to obtain ZUE12 antigen-positive human bone marrow MSCs. ZUE12 antigen-positive cells and negative cells were inoculated on culture dishes respectively, and placed in low-sugar DMEM medium containing 10% (V / V) fetal bovine serum at 37°C with a volume fraction of 5% CO 2 Culture in an incubator, and then change the medium every 3 days.
[0024] After 5 days of culture, the cells in the culture dish inoculated ...
Embodiment 2
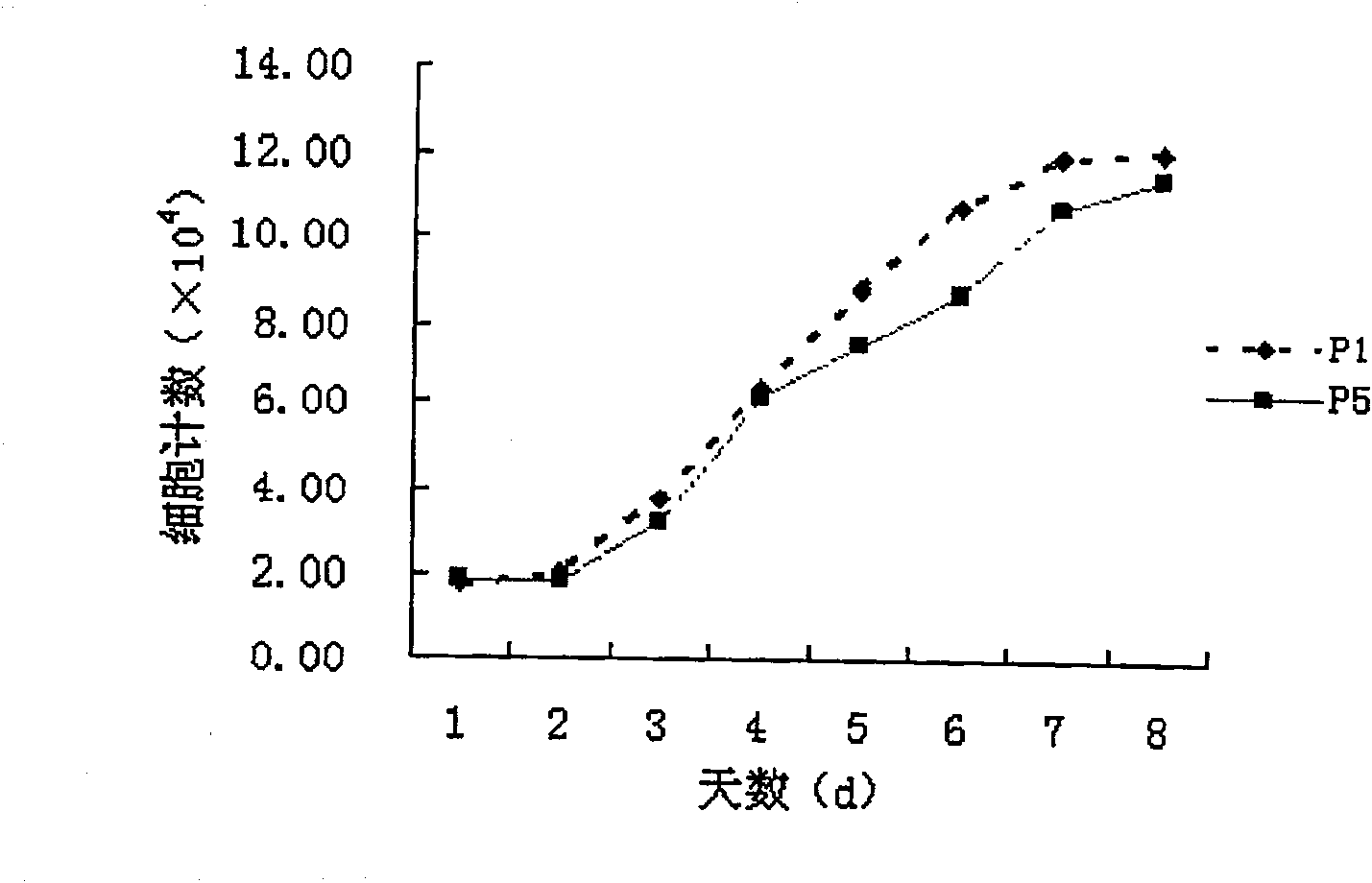
[0027] Analysis of the proliferation ability of positive cells obtained by forward immune sorting using ZUE12 as a marker
[0028] Take the ZUE12 antigen-positive human bone marrow MSCs first and fifth generation cells cultured in vitro, and press 2×10 4 Cells / well density were seeded in 24-well culture plates, cultured for 1, 2, 3, 4, 5, 6, 7, and 8 days, and cell kinetics analysis was performed. The culture has the following common characteristics: After the cells are subcultured, the cells are completely attached to the wall in about 10 hours, and the cell shape becomes a spindle cell again; the incubation period of the subculture is about 24 to 48 hours; days; after the end of the logarithmic growth phase, it enters the plateau phase; there is no significant difference between the growth of the fifth generation and the first generation cells, see figure 2 . Positive cells obtained by forward immune sorting with ZUE12 as a marker have good proliferative activity.
Embodiment 3
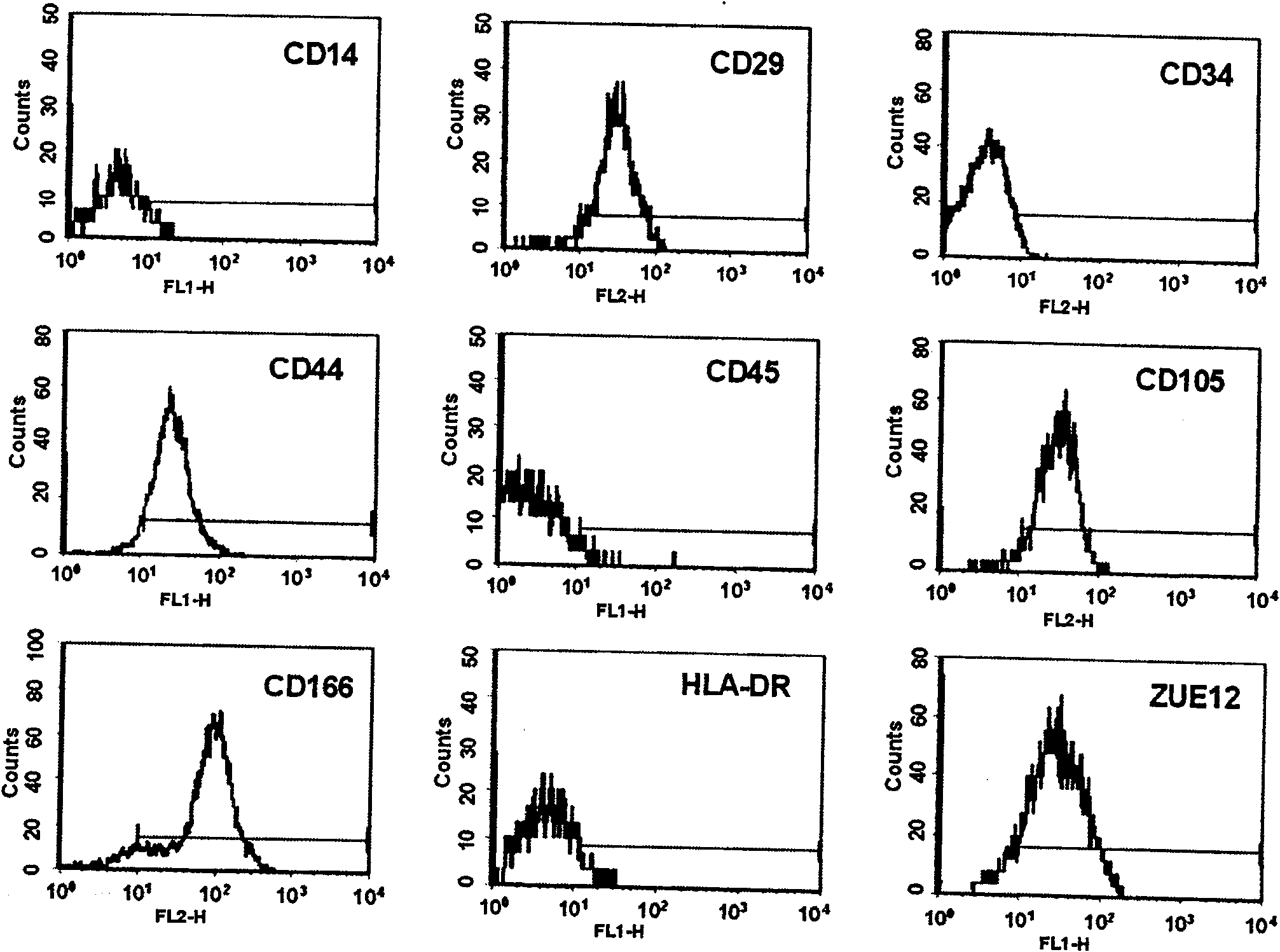
[0030] Phenotypic characteristics of positive cells obtained by forward immuno-sorting using ZUE12 as a marker
[0031] The first-generation and fifth-generation cells of human bone marrow MSCs positive for ZUE12 antigen cultured and passaged in vitro were cultured and passaged in vitro, and CD14-FITC, CD29-PE, CD34-PE, CD44-FITC, CD45-FITC, CD105-PE, CD166-PE, HLA-DR-FITC monoclonal antibody was used as a probe, and the expression of MSCs surface molecules was analyzed by flow cytometry. The results showed that CD29, CD44, CD166, and CD105 (SH2) positive markers showed a single peak, and the expressions of hematopoietic cell differentiation antigens CD14, CD34, CD45, and HLA-DR were all negative, see image 3 , image 3 Immunophenotypic analysis of positive cells obtained after forward immuno-sorting marked with ZUE12 and expanded to the fifth passage in vitro. The phenotypic characteristics of positive cells after forward immuno-sorting with ZUE12 are those of human bone m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com