Inhibitors of HSP90
A kind of CH2, low-level technology, applied in the field of benzimidazolone derivatives in the preparation of pharmaceutical compositions for the treatment of the disease, the pharmaceutical compositions for the treatment of the diseases, and the preparation of new intermediate compounds of the benzimidazolone derivatives, Can solve problems such as poor solubility, difficult formulation/application, difficult synthesis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0067]In the following preferred embodiments, general expressions may be replaced by corresponding more specific definitions provided above and below, thereby resulting in more preferred embodiments of the invention.
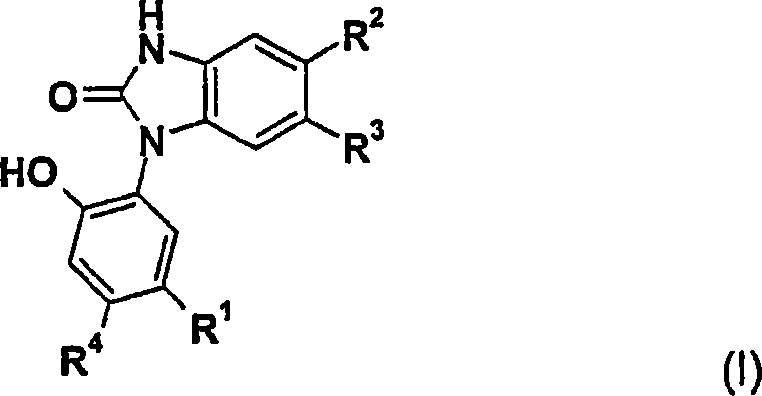
[0068] Preference is given to the use of compounds of formula (I) or (IA) or pharmaceutically acceptable salts thereof for the treatment of proliferative diseases.
[0069] Especially preferred is the use of a compound of formula (I) or (IA) or a pharmaceutically acceptable salt thereof in the treatment of proliferative diseases, wherein the disease to be treated is a disease dependent on Hsp90 and / or hsp90 client protein or an overexpression of Hsp90 tumor.
[0070] Also preferred is the use of a compound of formula (I) or (IA) or a pharmaceutically acceptable salt thereof in the preparation of a pharmaceutical preparation for the treatment of proliferative diseases, which pharmaceutical preparation comprises a compound of formula (I) and optionally a pharmaceu...
Embodiment 1
[0153] The synthesis of substituted anilines is carried out by nitrating the corresponding alkyl-resorcinols of formula (v) or 2,4-dimethoxy-acetophenone derivatives of formula (vi) to obtain formulas (iii) and 2,4-Dimethoxy-1-nitro-benzene derivatives of (iv). Friedel-Crafts acylation of 2,4-dimethoxy-1-nitro-benzene (vii) was performed using copper triflate as catalyst. Reduction of the corresponding The nitro derivatives (iii) and (iv) give 2,4-dimethoxy-5-substituted anilines. For simultaneous hydrogenation of nitro and keto groups, hydrochloric acid is added. 2,4-Dimethoxy-1-alkyl-benzenes (v) are obtained by hydrogenation of the corresponding 2,4-dimethoxy-acetophenones (vi).
[0154] Step 1.1: 5-Ethyl-2,4-dimethoxy-phenylamine
[0155] A solution of 1-ethyl-2,4-dimethoxy-5-nitro-benzene (Step 1.2) (6.1 g, 29 mmol) in ethanol (200 mL) was hydrogenated over Pd(C) (600 mg) for 1 h . The catalyst was filtered, the solvent was evaporated under reduced pressure and the ...
Embodiment 2
[0159]Example was synthesized from 5-chloro-2,4-dimethoxy-phenylamine and 1-fluoro-2-nitro-4-trifluoromethyl-benzene following a procedure similar to that described in the Synthetic Methods section 2 compounds.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap