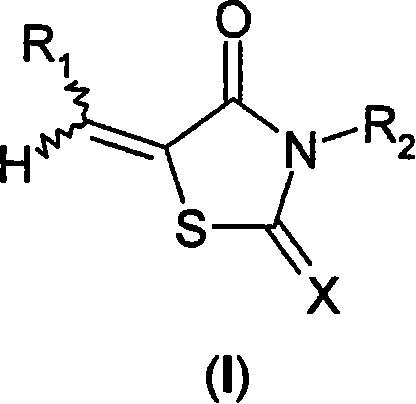
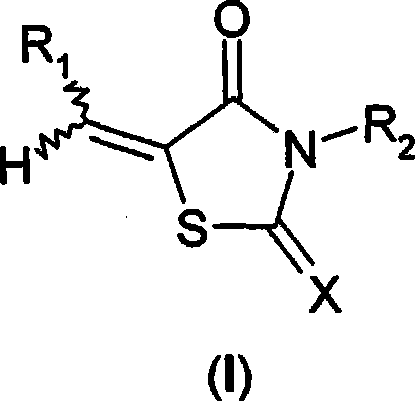
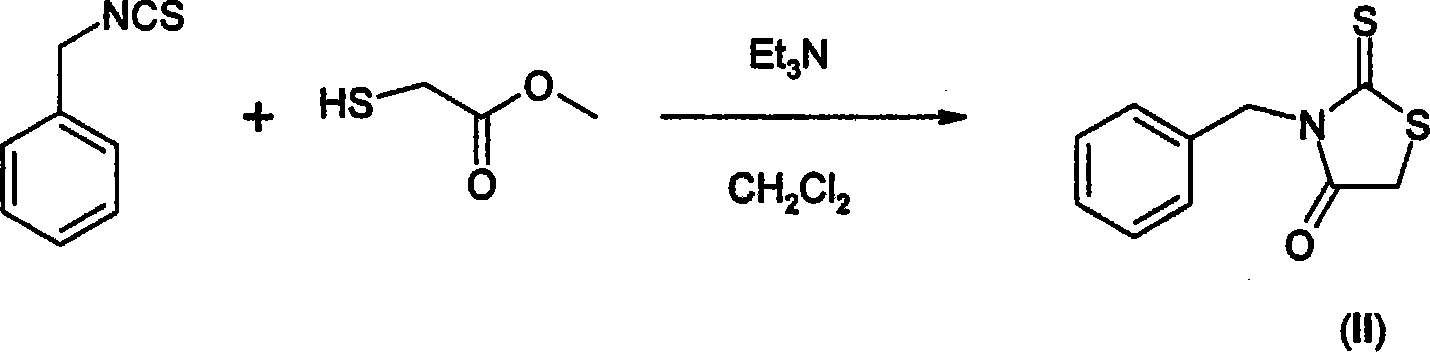
Use of thiazolidinone derivatives as antiangiogenic agents
A technology of thiazolidine and application, applied in the field of thiazolidinone compounds, can solve the problem that the mechanism of action is not completely clear and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0086] Example - 4-(5-formyl-furan-2-yl)-benzoic acid methyl ester
[0087] 5-Bromofural (2.43g, 13.9mmole), 4-(methoxycarbonyl)phenylboronic acid (2.50g, 13.9mmole), tris(dibenzylideneacetone)palladium (0) (192mg, 0.2 mmole) and potassium fluoride (2.42 g, 41.7 mmole) in 1,4-dioxane (100 ml) was added to a solution of tri-tert-butylphosphine in hexane (10% by weight, 101 mg, 0.5 mmole). After heating at 65-70°C for 4 hours, the mixture was cooled to room temperature and treated with dichloromethane (150ml). After stirring for 10 minutes, the mixture was filtered through celite, and the filtrate was concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel, eluting with ethyl acetate-hexane mixture (1:1), to give 4-(5-formyl-furan-2-yl)-benzoic acid methyl ester (2.6 g, 81 %Yield).
[0088] 1 H NMR (300MHz, CDCl 3 ): δ9.70(s, 1H), 8.10(d, 2H), 7.90(d, 2H), 7.35(d, 1H), 6.95(d, 1H), 3.98(s, 3H).
[0089] General Process B
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com