Method for treatment of movement disorders
A technology for dyskinesia and dystonia, applied in the direction of anhydride/acid/halide active ingredients, nervous system diseases, neuromuscular system diseases, etc., which can solve problems such as rebound, gastroesophageal erosion, and increased risk of liver toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Xyrem A single-patient clinical trial for myoclonus after severe hypoxia. The patient was a 37-year-old woman who suffered an accidental anesthesia. After remaining in a coma, she woke up and recovered gradually, however, she was completely disabled due to severe myoclonic spasms that affected her voice, head, proximal arms , legs and torso. On clinical examination, she had positive myoclonus (active spasm) and negative myoclonus (postural deviation). Her myoclonus had been treated with phenobarbital, zonisamide, clonazepam, and levetiracetam without significant improvement. Before the trial, she was treated with clonazepam and levetiracetam. She is allergic to penicillin, has no other known drug allergies, and is otherwise in good general health.
[0077] Case Reports
[0078] Open-label GHB in a single patient with severe debilitating alcohol-responsive posthypoxic myoclonus that is refractory to treatment with standard antimyoclonus agents , Blind grading ...
Embodiment 2
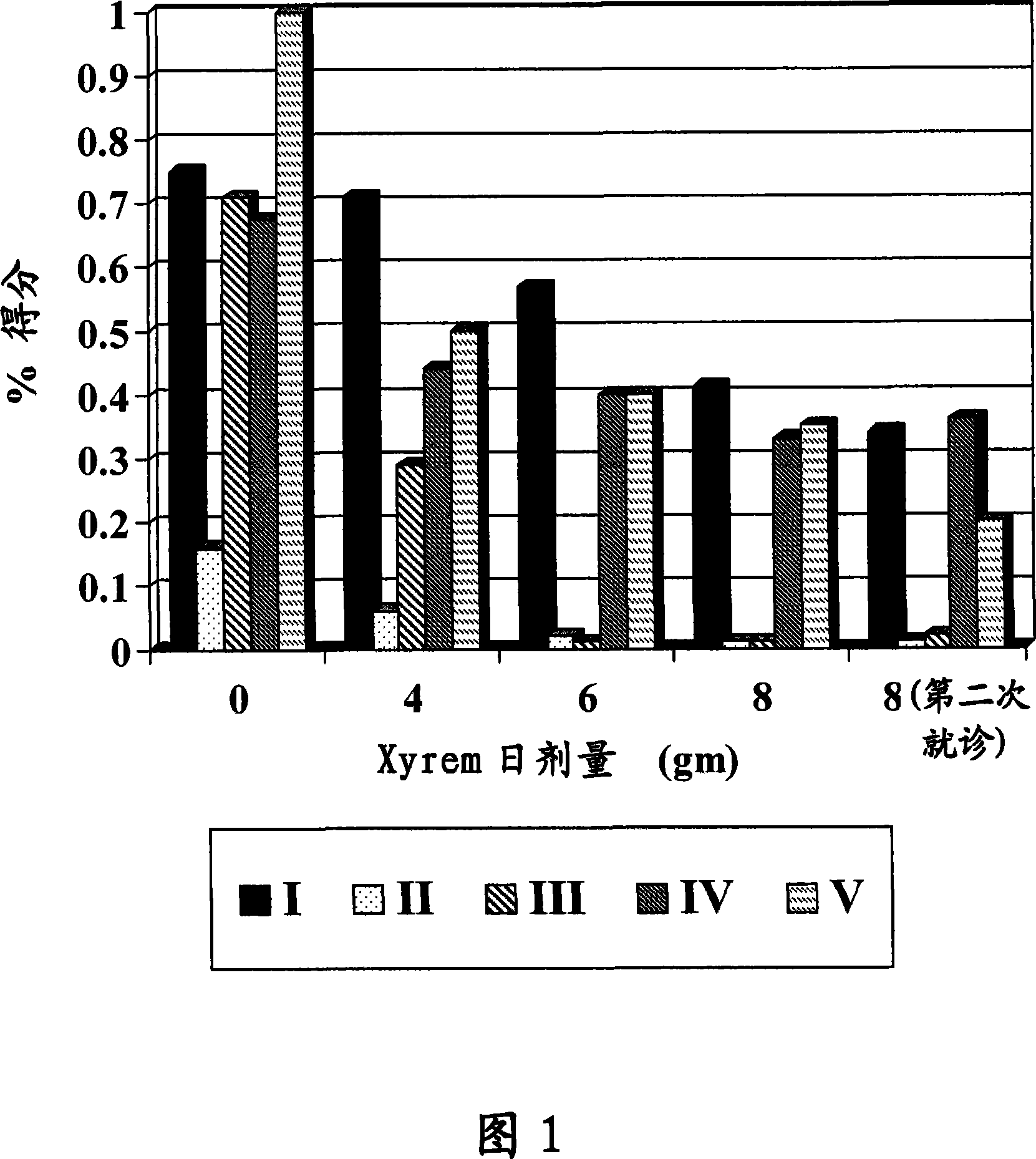
[0097] In this prophetic example, approximately 20 patients will undergo a short-term double-blind, placebo-controlled regimen followed by an open-label extension. The protocol will require dose adjustments of up to 6.125 gm / day during the double-blind period and up to 9 gm / day of options during the open-label period.
[0098] experimental design
[0099] This study is a double-blind, randomized, placebo-controlled, parallel-group, dose-variation trial of dystonic GHB. The study population included patients with clinically apparent myoclonus-dystonia.
[0100] The primary goals include: 1) assessing the safety and tolerability of GHB in dystonic patients, and 2) assessing the efficacy of GHB in the treatment of dystonia. Secondary objectives were: 1) To assess the effect of GHB dose on dystonia.
[0101] The duration of the double-blind portion of the study was 8 weeks. The duration of the fixed-dose portion of the study was 8 weeks. The duration of the dose change port...
Embodiment 3
[0110] patients and methods
[0111] Five patients participated in the trial in the Movement Disorders Division of Columbia University Medical Center in the fall of 2004. All patients had hyperkinetic dyskinesia (defined as a marked change in the patient) responsive to ethanol, and all were refractory to treatment with conventional drugs or could not tolerate them. The Medical Center's Institutional Review Board approved the trial, and written and oral informed consent was obtained from all patients prior to participation in the trial. Prominent clinical features appear below and are summarized in Table 1.
[0112] Table 1: Clinical characteristics of patients with alcohol-responsive dyskinesia
[0113]
Pt #
M / F
age
Dx
t(year)
Current Rx
Past Rx
1
F
37
PHM
6
Levetiracetam 2,5...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap