Sars vaccines and methods to produce highly potent antibodies
A technology of vaccines and antibodies, which is applied in the fields of preparation methods of peptides, chemical instruments and methods, medical preparations containing active ingredients, etc., and can solve problems such as unclear efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
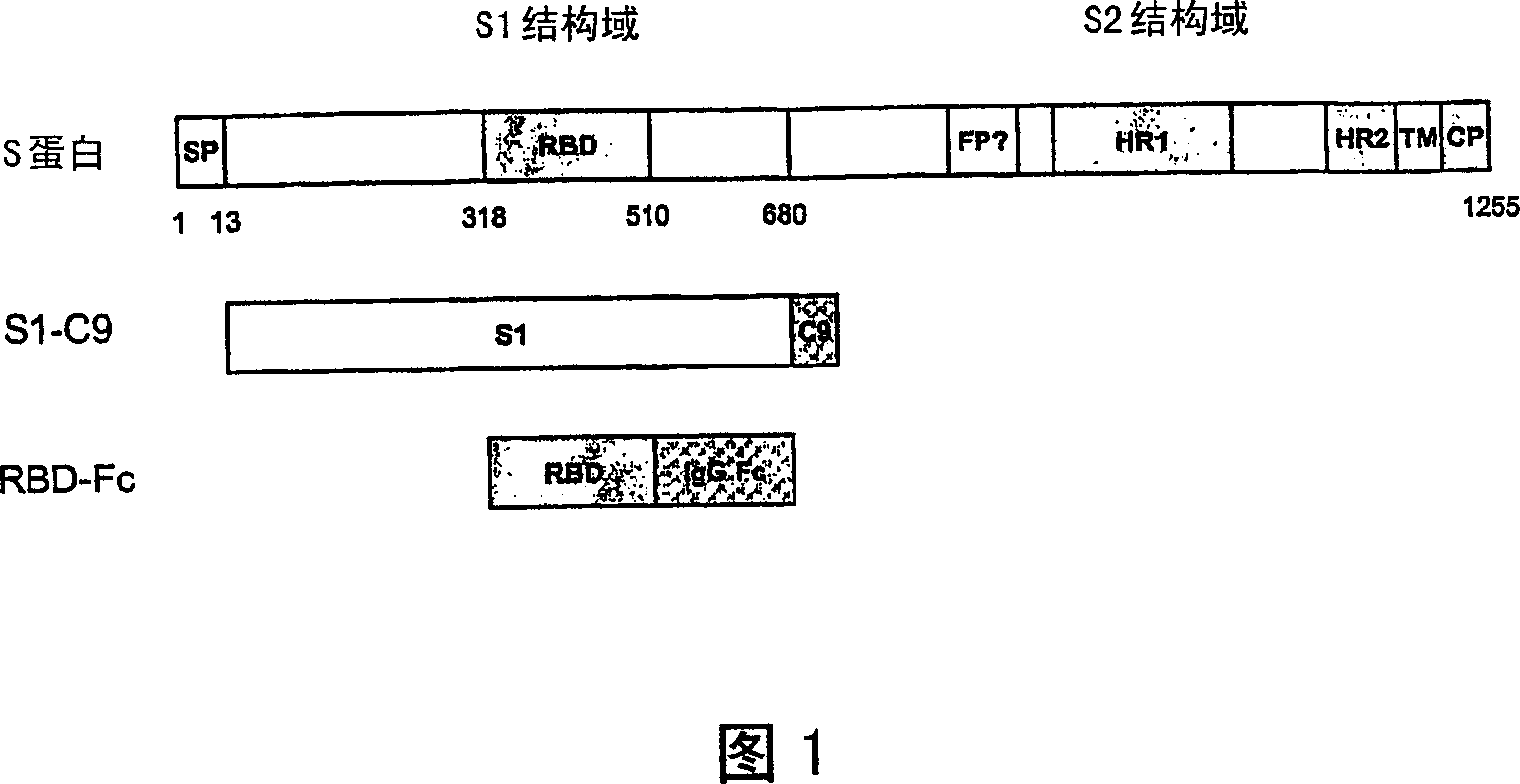
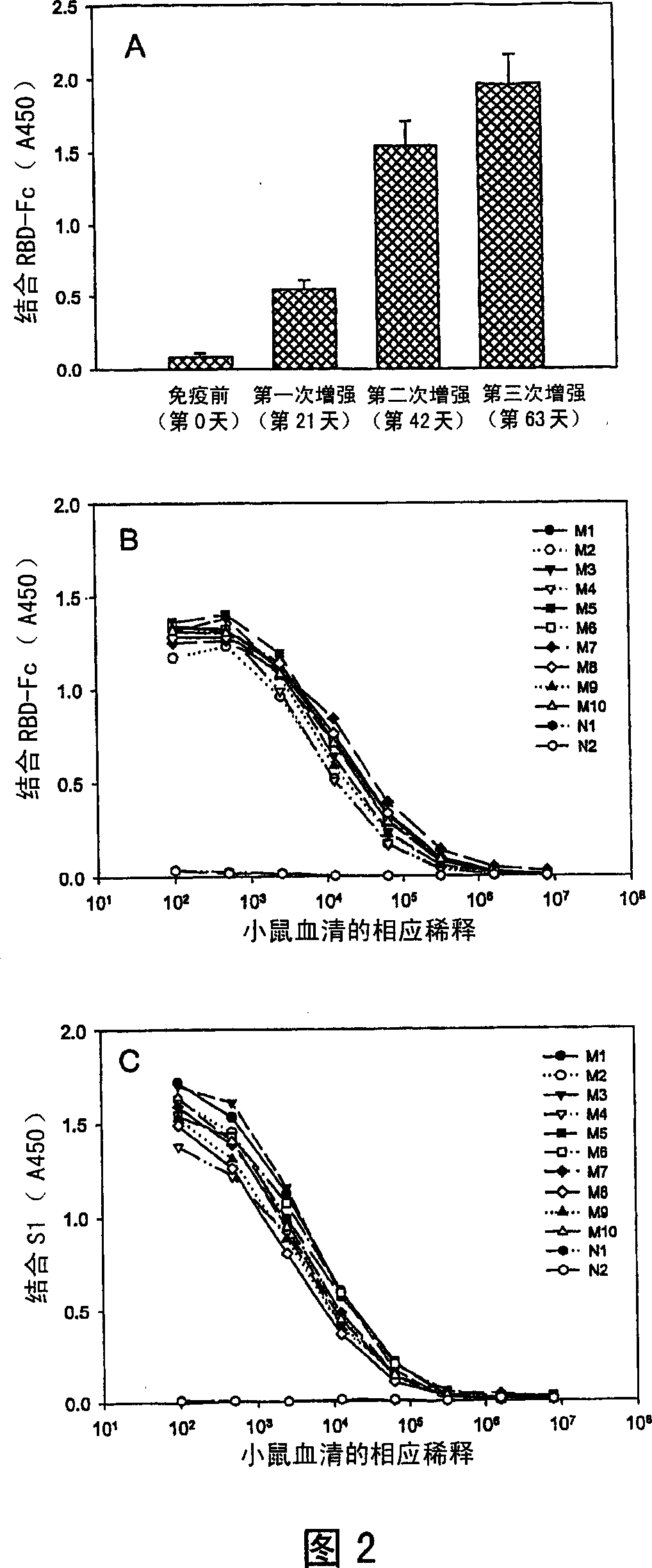
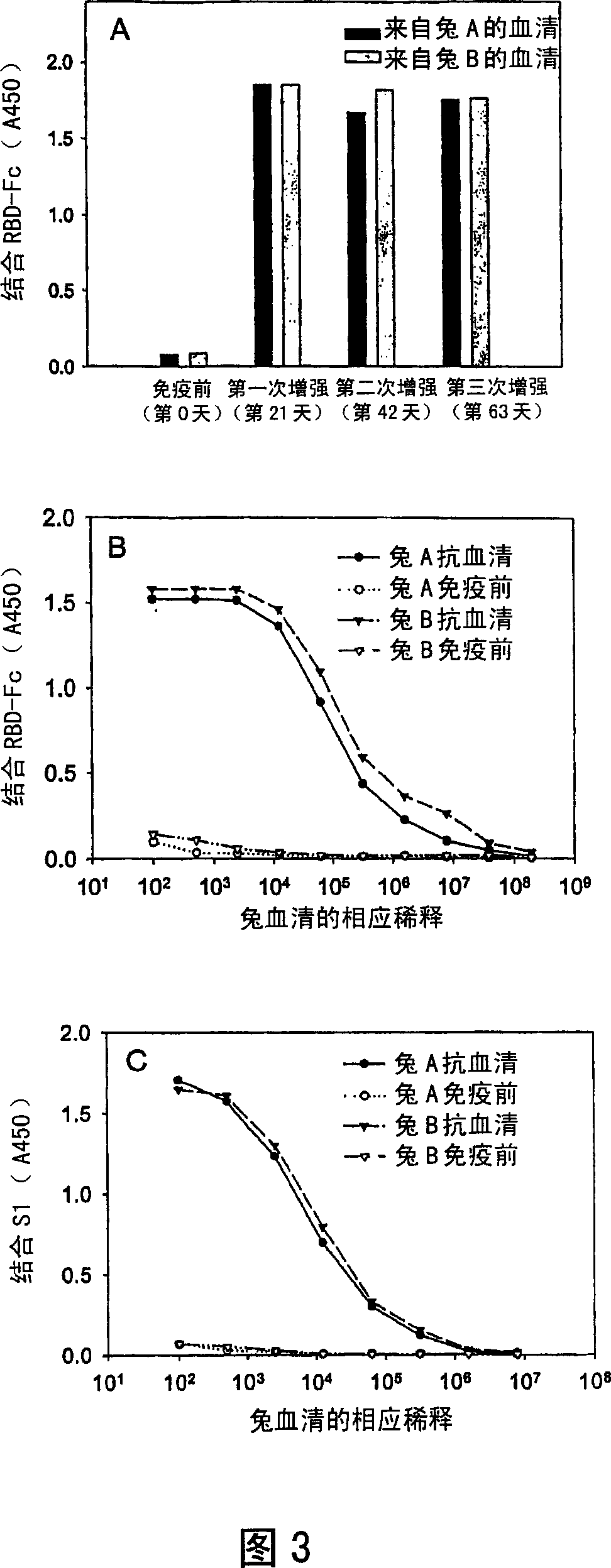
[0030] The present invention provides a vaccine comprising an effective amount of isolated polypeptides and recombinant proteins containing the receptor binding domain (RBD) of SARS-associated coronavirus spike protein or its function fragment. In one embodiment, suitable adjuvants are used with the vaccines described herein. In another embodiment, the vaccine is conjugated.
[0031] As used herein, a functional fragment is the portion of the RBD that performs the described function. In one embodiment, the function is to bind a receptor.
[0032] Peptide or polypeptide or protein with RBD sequence
[0033] The present invention provides an isolated peptide or polypeptide or protein comprising a receptor binding domain sequence or a functional fragment thereof in the spike protein of severe acute respiratory syndrome-associated coronavirus, which can be used for preventing severe acute respiratory syndrome-associated coronavirus Vaccine for coronavirus infection.
[0034] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com