Uses of rifamycins
A technology of rifamycin and erythromycin, which is applied in the treatment of osteomyelitis and foreign body infection in patients who need treatment, and can solve the problem of damage to the phagocytic bactericidal ability of granulocytes at the implantation site
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
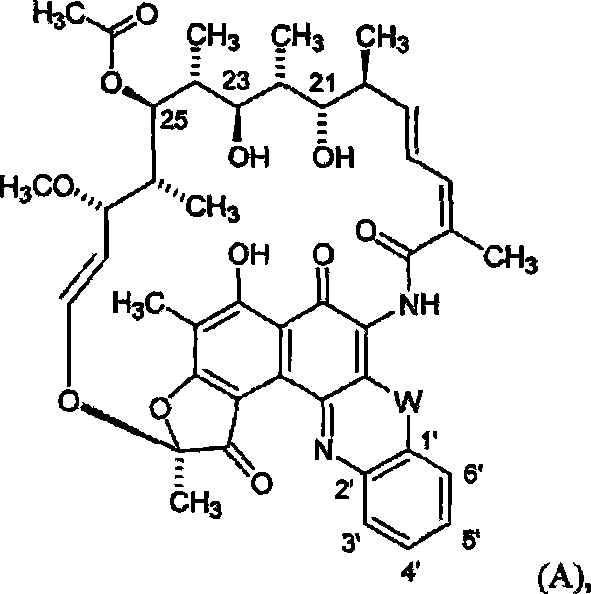
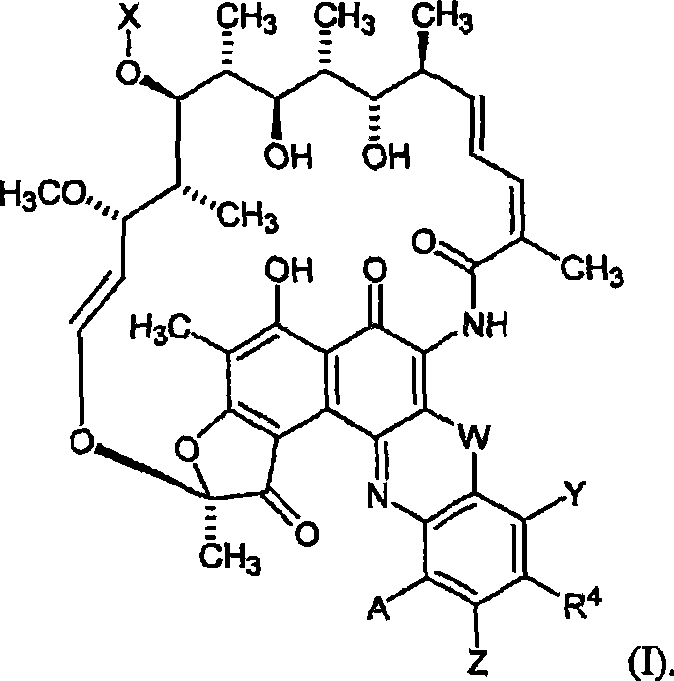
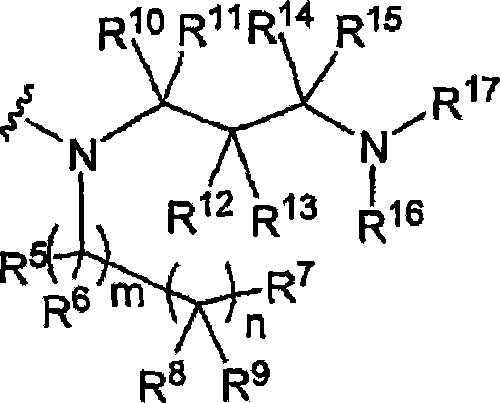
[0287] Example: Efficacy of 25-O-desacetyl-3'-hydroxy-5'-(4-methylpiperazinyl)benzoxazine rifamycin against Staphylococcus aureus in a foreign body infection model
[0288] We evaluated 25-O-desacetyl-3′-hydroxy-5′-(4-methylpiperazinyl)benzoxazine rifamycin (compound No. 86) alone and in combination with levofloxacin (LVX). Use, against Staphylococcus aureus in an established foreign body infection model (JID 1982; 146:486).
[0289] Methods: Four Teflon cages were implanted in the ventral side of guinea pigs. After two weeks, use 2 x 10 4 Cfu Staphylococcus aureus ATCC 29213 was inoculated into the cages. 24 hours after infection, the animals were treated by intraperitoneal injection every 12 hours for four consecutive days (see Table 4). Five days later, the tissue fluid in the cage was aspirated and cultured to detect floating Staphylococcus aureus. Then the cage was taken out, vortexed and incubated in broth medium at 37°C for 12h to detect adherent Staphylococcus aure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com