Compositions comprising nanoparticulate meloxicam and controlled release hydrocodone
A nanoparticle and release composition technology, applied in the direction of nanomedicine, nanotechnology, nanotechnology, etc., can solve problems such as addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
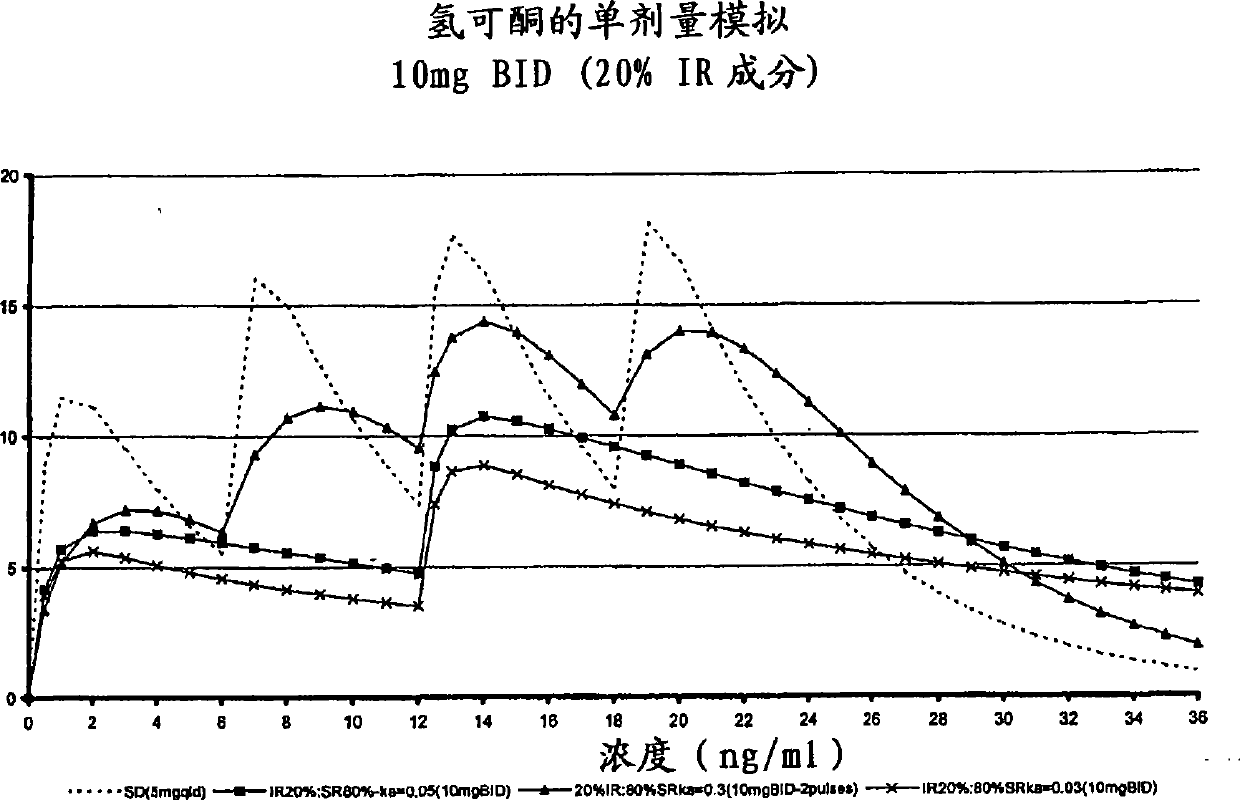
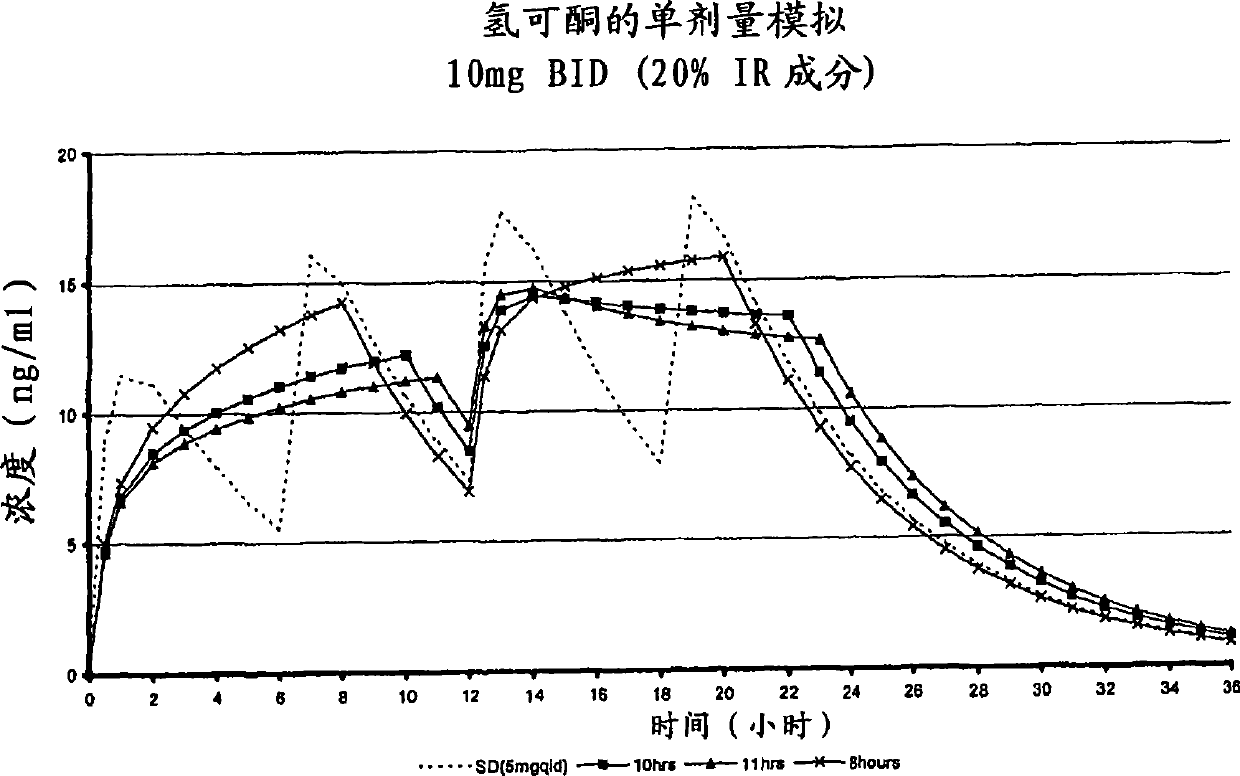
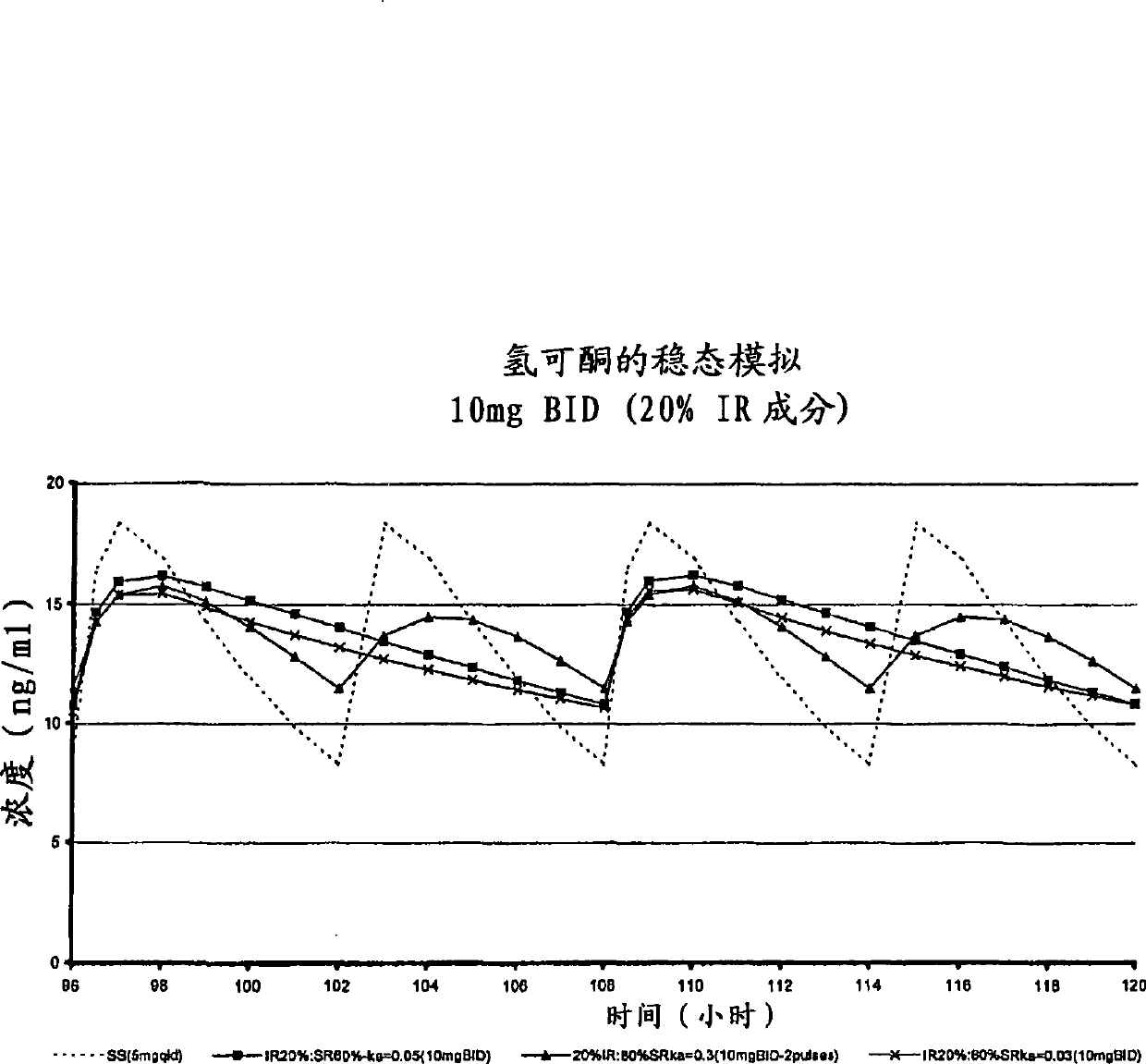
[0264] The purpose of this example is to describe a multiparticulate modified release composition comprising hydrocodone that can be used in the binding composition of the present invention.
[0265] A multiparticulate modified release hydrocodone composition according to the invention having an immediate release component and a modified release component (with a modified release coating) was prepared according to the formulations shown in Tables 1 and 2.
[0266] Table 1
[0267] Immediate release ingredient hydrocodone solution
[0268]
[0269] Table 2
[0270] Modified release ingredient hydrocodone solution
[0271]
[0272] In these exemplary hydrocodone formulations, sugar spheres (30 / 35 mesh) are provided as inert cores which serve as carriers for the active ingredient and other excipients present in the formulation. The amount and size of the sugar spheres is chosen to reflect the requirement that the average diameter of the produced multiparticulates be in t...
Embodiment 2
[0298] The purpose of this example was to prepare meloxicam nanoparticle dispersions stabilized with various surface stabilizers.
[0299] An aqueous dispersion formed of 5 wt % meloxicam (Unichem Laboratories, Ltd.) and 1 wt % stabilizer (see Table 9 below) was added to a 10 cc batch chamber equipped in milling systems (Elan Drug Delivery, Inc., King of Prussia, PA; see, eg, US Patent No. 6,431,478 entitled "SmallScale Mill"). For all formulations the following Milling system parameters: milling speed = 5500 rpm; total milling time = 1 hour; type of polymeric milling media = PolyMill™ 200 (The Dow Chemical Co.); and media loading sufficient to carry out the above process.
[0300] The resulting milled dispersions were analyzed for particle size using a Horiba LA-910 Particle Size Analyzer (Horiba Instruments, Irvine, CA). The results obtained are shown in Table 9 below. In the table below, the D50 value is the particle size below which 50% of the active agent particles f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average diameter | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com