Fusion polypeptide for inhibiting neurotransmitter secretion and method for delivering it
A technology that combines polypeptides and neurotransmitters, applied in chemical instruments and methods, hybrid peptides, nervous system diseases, etc., can solve the problems that the applicability of the biologically active substances to be delivered has not been clearly confirmed, and achieve Efficiency-promoting and secretion-inhibiting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of PTD-SBD (VBD) conjugate
[0056] (1) Preparation of PTD-SBD (VBD) conjugates
[0057] YARVRRRGPRRGGGEIDTQNRQIDRIMEKAQANKTRIDEANQRATKMLGSG (PTD-GGG-SBD polypeptide)
[0058] According to the method of solid-phase synthesis, a fusion polypeptide (PTD-GGG-VBD polypeptide) whose amino acid sequence is YARVRRRGPRRGGGNRRLQQTQAQVDEVVDIMRVNVDKVLERDQKLSELDDRADALQAGASQFETSAAKLKR was synthesized using a semi-automatic synthesizer (Peti-Syzer Model PSS-510). In order to achieve this purpose, 0.1 mmol of Rink Amide MBHA resin was placed in a standard reactor, and 0.5 mmol of activated Fmoc-G and Fmoc-R (Fmoc-G and Fmoc-R) were added to the reactor. Fmoc-R is the first C-terminal amino acid of the polypeptide to be synthesized), and then the synthesis of the polypeptide is started. In order from C-terminal amino acid to N-terminal amino acid, 0.5 mmol of each corresponding amino acid was condensed 3 times. Using dimethylformamide (DMF) containing 20%...
Embodiment 2
[0061] Example 2: Preparation and purification of expression vectors for fusion polypeptides using microbial expression systems
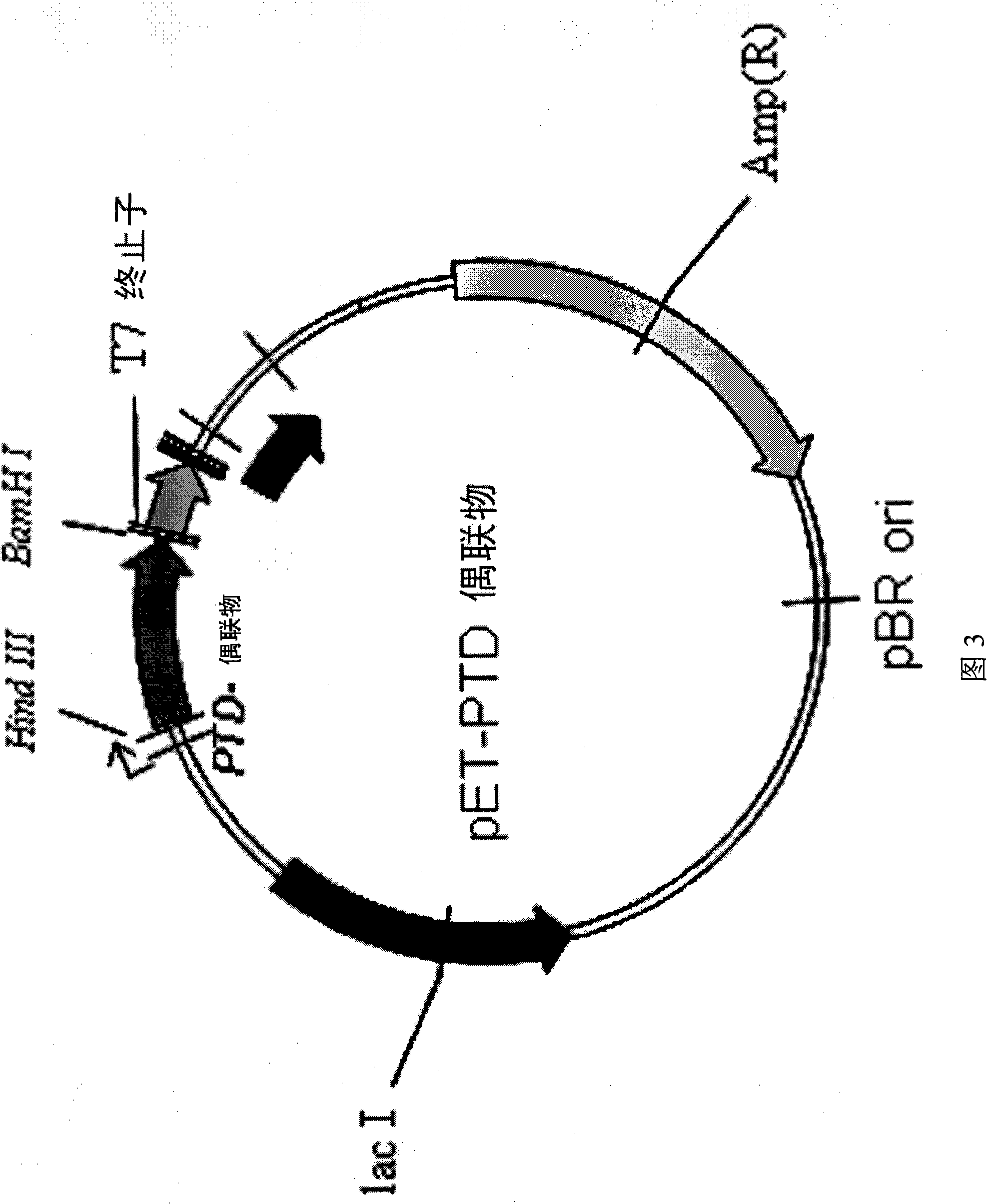
[0062] (1) Preparation of expression vector
[0063] To construct the base sequence encoding the fusion polypeptide, an N-terminal Hind III cleavage site and a C-terminal BamH I cleavage site were set in pUC19 (purchased from Invitrogen), thereby constructing a template. In order to bind the polyhistidine tag, which enables high expression and easy purification of the fusion polypeptide, to the base sequence encoding the highly expressed protein, the template was isolated using restriction enzymes HindIII and BamH I, and purified using Auiaquick reagent box for purification. The expression vector pPET was treated with restriction enzymes HindIII and BamH I, and purified with Auiaquick purification kit. The purified expression vector is then cloned into the above-prepared base sequence template encoding the fusion polypeptide, thus preparing a re...
Embodiment 3
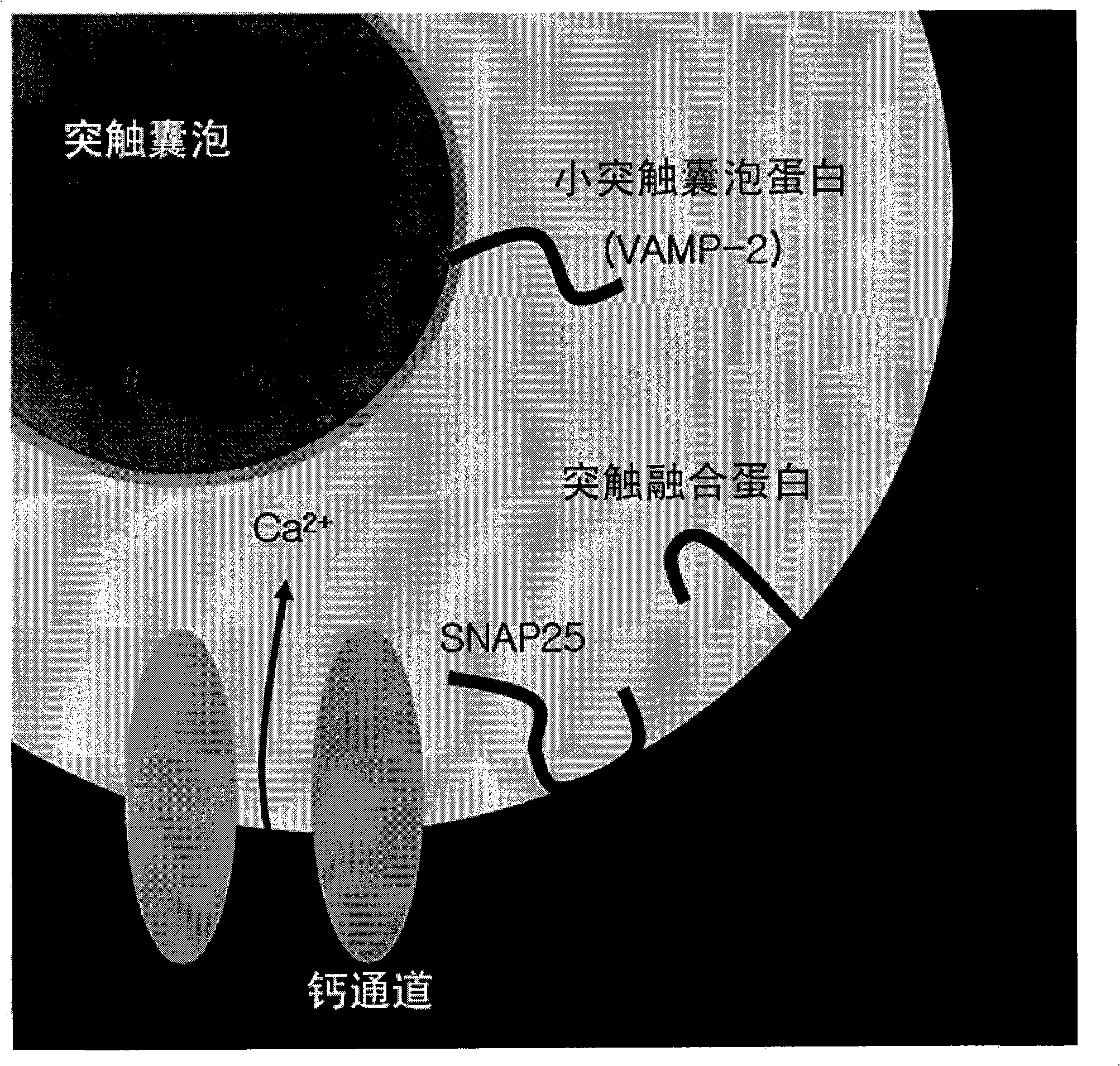
[0066] Example 3: Effect of Fusion Polypeptide Inhibiting Neurotransmitter Secretion in Test Tubes.
[0067] The effects of the fusion polypeptides prepared in Examples 1 and 2 on inhibiting neurotransmitter secretion were analyzed. To compare the activities, two fragments were prepared separately for SBD and VBD. The fragment samples are as follows:
[0068] Sample 1: SBD (SEQ ID NO: 4)
[0069] Sample 2: VBD (SEQ ID NO: 5)
[0070] Sample 3: Hph-1-GGG-SBD (SEQ ID NO: 14)
[0071] Sample 4: Hph-1-GGG-VBD (SEQ ID NO: 15)
[0072] Sample 5: Tat-GGG-SBD (SEQ ID NO: 16)
[0073] Sample 6: Hph-1-GGG-SBDF1 (SEQ ID NO: 17)
[0074] Sample 7: Hph-1-GGG-SBDF2 (SEQ ID NO: 18)
[0075] Sample 8: Hph-1-GGG-VBDF1 (SEQ ID NO: 19)
[0076] Sample 9: Hph-1-GGG-VBDF2 (SEQ ID NO: 20)
[0077] Hph-1-GGG-SBD (SEQ ID NO: 14)
[0078] YARVRRRGPRRGGGEIDTQNRQIDRIMEKAQANKTRIDEANQRATKMLGSG
[0079] Hph-1-GGG-VBD (SEQ ID. NO.: 15)
[0080] YARVRRRGPRRGGGNRRLQQTQAQVDEVVDIMRVNVDKVLERDQKLSEL...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap