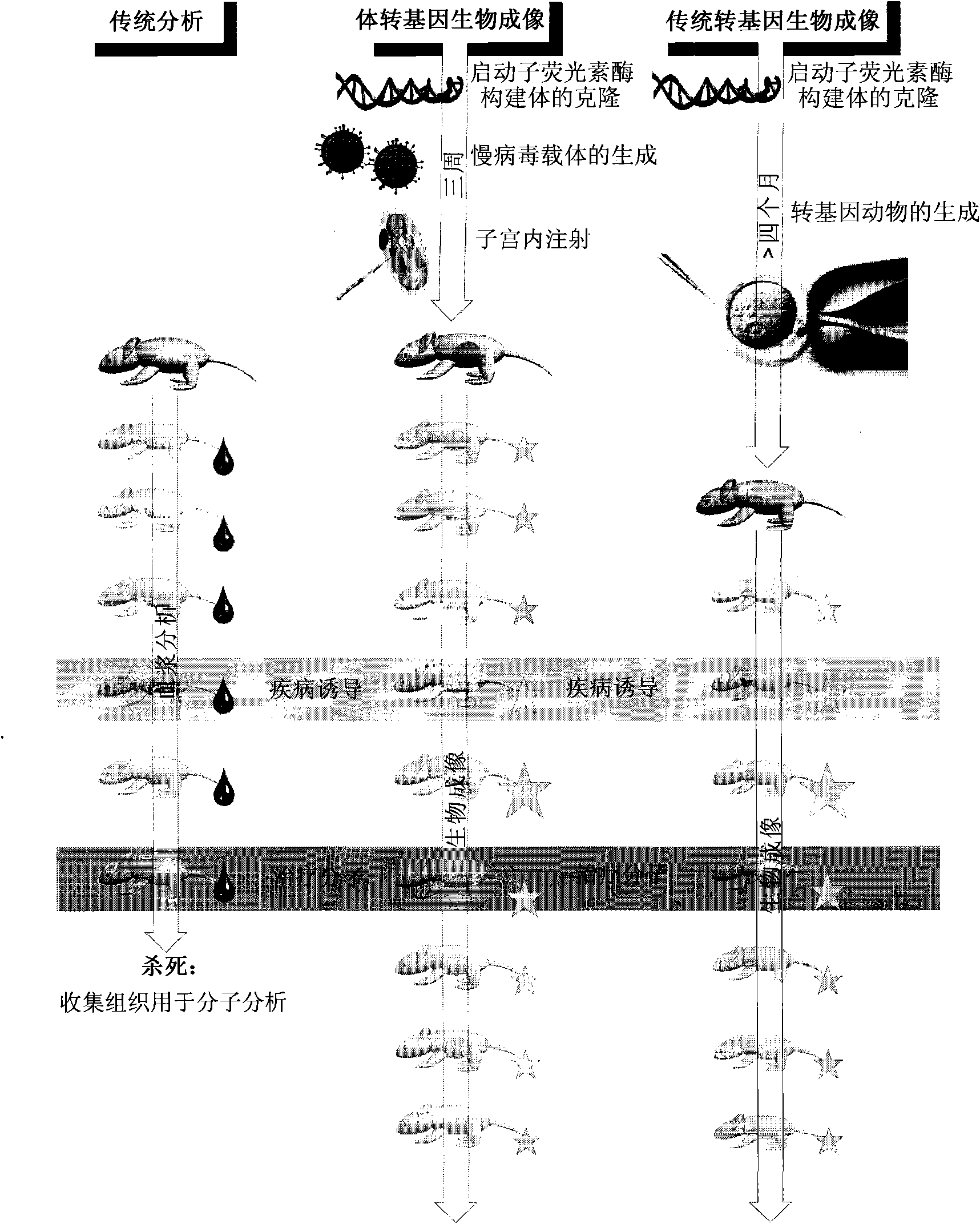
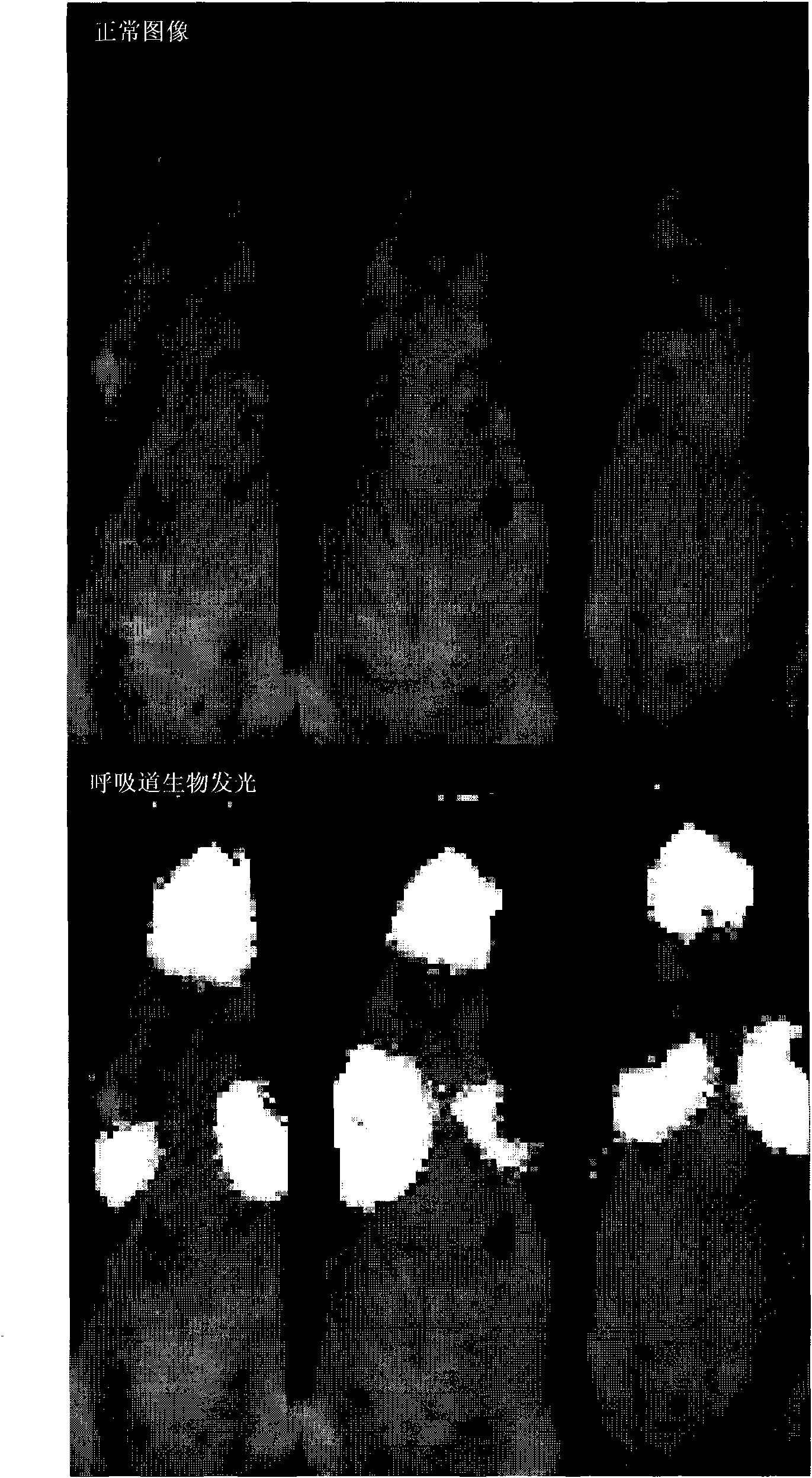Somatotransgenic bioimaging
A technology for transgenic animals and gene products, which can be used in genetic engineering, biochemical equipment and methods, and the determination/inspection of microorganisms, which can solve problems such as limitations and time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Experiments were performed to determine the possibility of achieving long-term tissue-specific transgene expression in mice.
[0111] Vector Preparation and Validation
[0112] gp64-pseudotypes for long-term analysis were prepared as described by Seppen et al. luciferase vector and vsvg-pseudotyped luciferase vector.
[0113] The lentivirus was prepared as follows: Inoculate 2 × 10 7 293T production cells. The following day, the following amounts of plasmid DNA were mixed in OptiMEM (Invitrogen, Paisley, UK) in each T-150 flask to a final volume of 5 ml: vector construct (pHR.SINcpptSEW) 40 μg, pMDG.2 / pHCMVwhvGP64 10 μg, pCMVΔ8.7430 μg. Polyethylenimine (PEI, 25 kDa) (Sigma, Poole, UK) was added to 5 ml OptiMEM to a final concentration of 2 μM and filtered through a 0.22 μm filter. The DNA was added dropwise to the PEI solution and incubated at room temperature for 20 minutes. The DNA / PEI solution was added to the 293T cells and incubated at 37°C, 5% CO 2 Incuba...
Embodiment 2
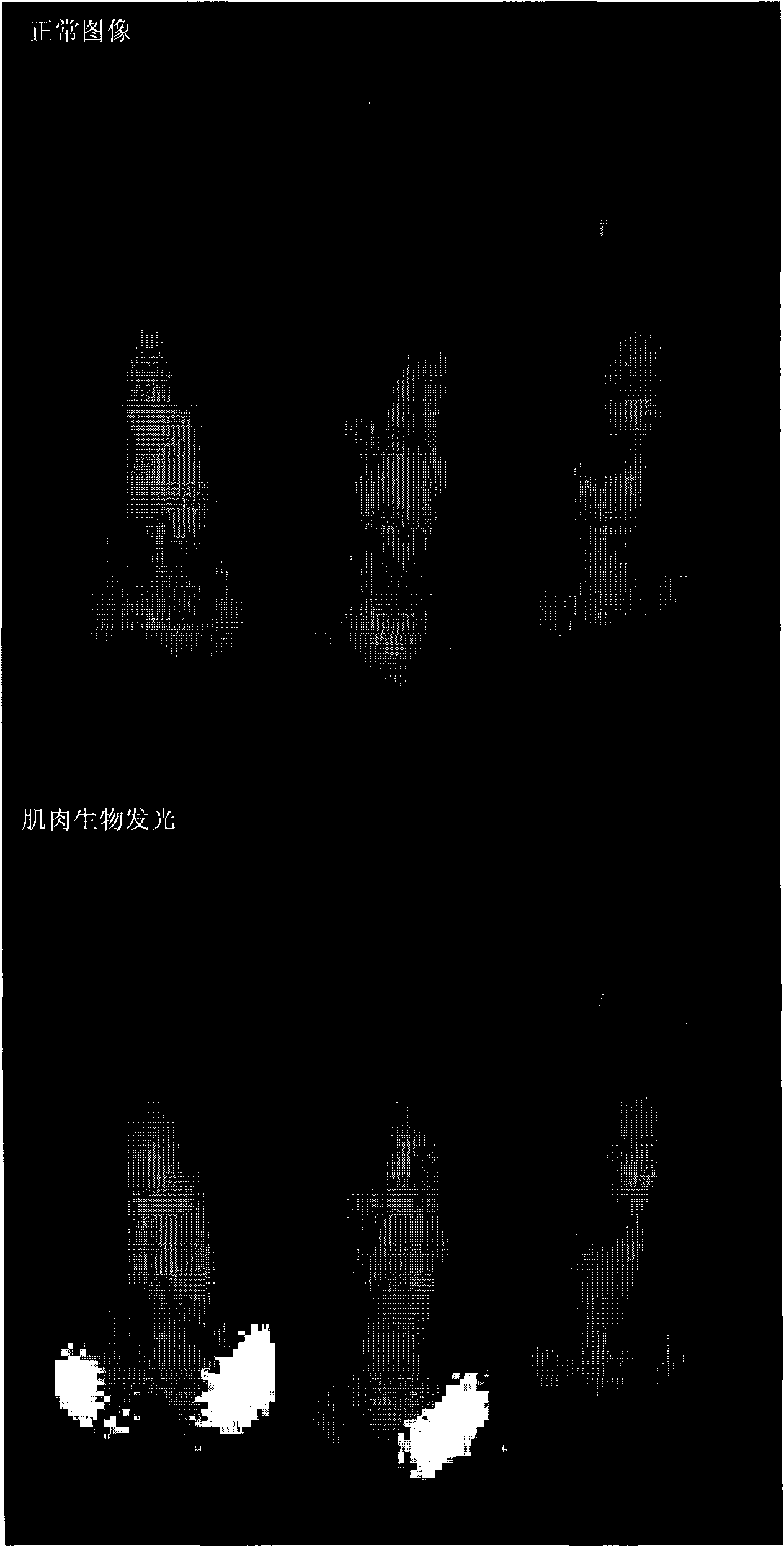
[0122] To assess long-term transgene expression, a single dose of gp64 / HIV luciferase (approximately 3 × 10 7 iu). Mice were biologically imaged over a year and older and luciferase bioluminescence was compared to controls (n=2). In vivo luciferase bioimaging was performed as described in Example 1. Luciferase expression was significantly higher than control throughout the analysis and persisted throughout the study ( Figure 6 ). The results demonstrate that overt expression can be detected for up to one year after dosing.
Embodiment 3
[0124] Genetic bioeffectors useful in animal models were tested in vitro (Examples 3-5).
[0125] NIH-3T3 cells were transfected with a plasmid containing a TGF-β response element driving luciferase expression. These cells were then transduced with a retroviral vector expressing TGF-β3. The SBE4 response element is specific for TGF-β activation through smad2 / 3-mediated transcriptional activation. This Smad activation can be further characterized as Smad2-specific transcriptional activation using ARE response elements together with the Xenopus Fast-1 trans-effector (a single ARE is specific for Samd2 / 3 only). The BMP-specific response elements are activated by activation of Smad1 / 5 / 8 and should not respond to activation of TGF-β3. Finally, Samd7 is a suppressor Smad known to be upregulated in a negative feedback loop to TGF-β3 activation. The experiment was carried out as follows:
[0126] Press 1×10 6Cells / well were prepopulated with NIH-3T3 cells and transfected with 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com