An anti-cancer cytotoxic monoclonal antibody
A monoclonal antibody, cytotoxic technology, applied in the direction of antibodies, fusion cells, radioactive carriers, etc., can solve the problem of insufficient cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Hybridoma production--hybridoma cell line AR92A271.7
[0100] According to the Budapest Treaty, the hybridoma cell line AR92A271.7 was deposited in the International Depository of Canada (the International Depository Authority of Canada, IDAC) on May 29, 2007, and the Bureau of Microbiology, Health Canada (Bureau of Microbiology, HealthCanada) (Canada, Ma 1015 Arlington Street, Winnipeg, Nitoba, R3E 3R2), accession number 290507-04. Pursuant to 37 CFR 1.808, the depositor warrants that, at the time of grant of the patent, all constraints imposed on the public availability of the deposited material are irrevocable. If the depositary cannot release a viable sample, replace the deposit.
[0101] To generate hybridomas producing anticancer antibody AR92A271.7, single cell suspensions of frozen human lung adenocarcinoma tumor tissue (Genomics Collaborative, Cambridge, MA) were prepared in PBS. Prepare IMMUNEASY by mixing gently TM (Qiagen, Venlo, The Netherlands) adjuvant...
Embodiment 2
[0108] in vitro binding
[0109] AR92A271.7 monoclonal antibody was produced by growing hybridomas in CL-1000 flasks (BD Biosciences, Oakville, ON), harvested and reseeded twice / week. Standard antibody purification steps were followed using Protein G Sepharose 4 Fast Flow (Amersham Biosciences, Baie d'Urfe, QC). It is within the scope of the invention to use humanized, deimmunized, chimeric or murine monoclonal antibodies.
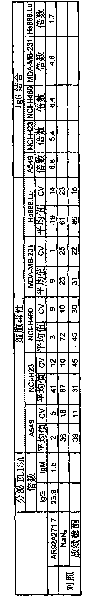
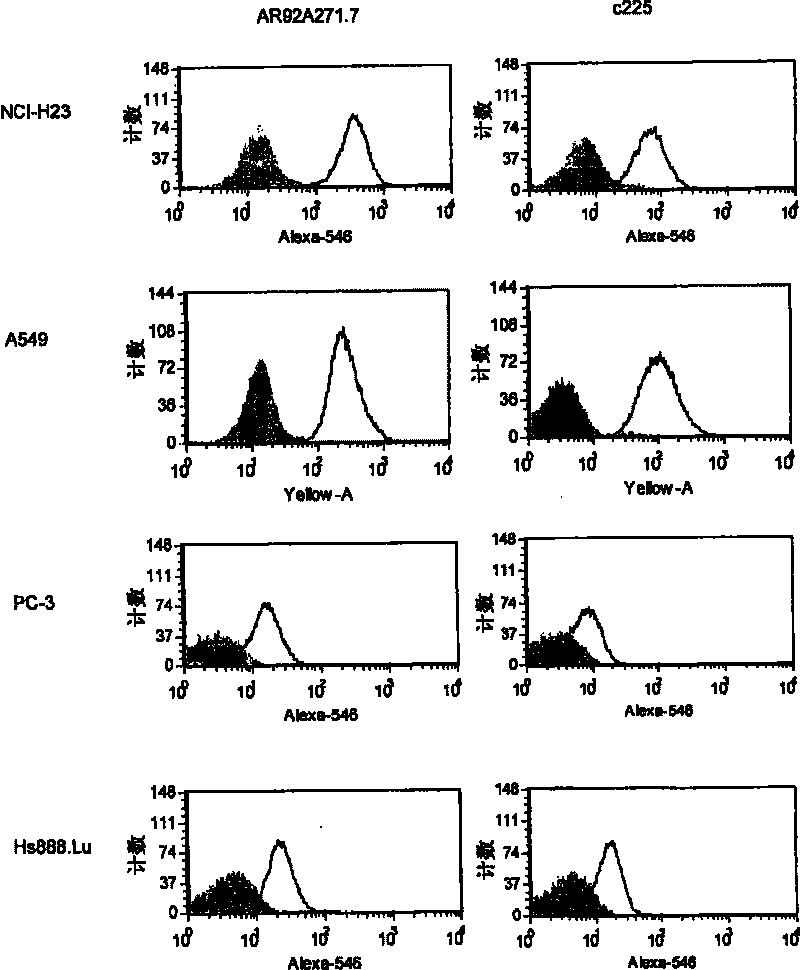
[0110] The relationship between AR92A271.7 and lung (A549, NCI-H23, NCI-H322M, NCI-H460 and NCI-H520), colon (Lovo), breast (MDA-MB-231), pancreas ( Combination of BxPC-3), prostate (PC-3) and ovarian (OVCAR-3) cancer cell lines, and non-cancer cell lines from skin (CCD-27sk) and lung (Hs888.Lu). All cell lines were obtained from the American Type Tissue Collection (ATCC, Manassas, VA).
[0111] By initially using DPBS (Ca-free ++ and Mg ++ ) to wash the cell monolayer and prepare the cells for FACS. Cells were then removed from their cell culture pl...
Embodiment 3
[0114] In vivo tumor experiments using A549 cells
[0115] Example 1 demonstrates that AR92A271.7 has anticancer properties against human lung cancer cell lines. To demonstrate in vivo efficacy against human lung cancer cell lines, AR92A271.7 was tested in the A549 lung cancer xenograft model. refer to Figure 4 and 5 , 6-8 week old female SCID mice were implanted with 1 million human lung cancer cells (A549) in 100 microliters of PBS solution by subcutaneous injection at the right loin. The mice were randomly divided into two treatment groups, 10 in each group. On the day after implantation, in a solution containing 2.7mM KCl, 1mM KH 2 PO 4 , 137mM NaCl and 20mM NaCl 2 HPO 4 After dilution of the diluent from the stock concentrate, each group was administered intraperitoneally with 20 mg / kg AR92A271.7 detection antibody or buffer control in a volume of 300 microliters. The antibodies and control samples were then administered weekly for the duration of the study. Tum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com