Small molecule compound having an IDO1/TDO double target, preparation method and applications thereof
A small molecule compound, IDO1 technology, applied in the fields of medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc., can solve the problem of no relevant articles reported, no IDO1/TDO dual target small molecule compounds have been found, etc. Achieve the effect of reducing immune evasion, good application prospects, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
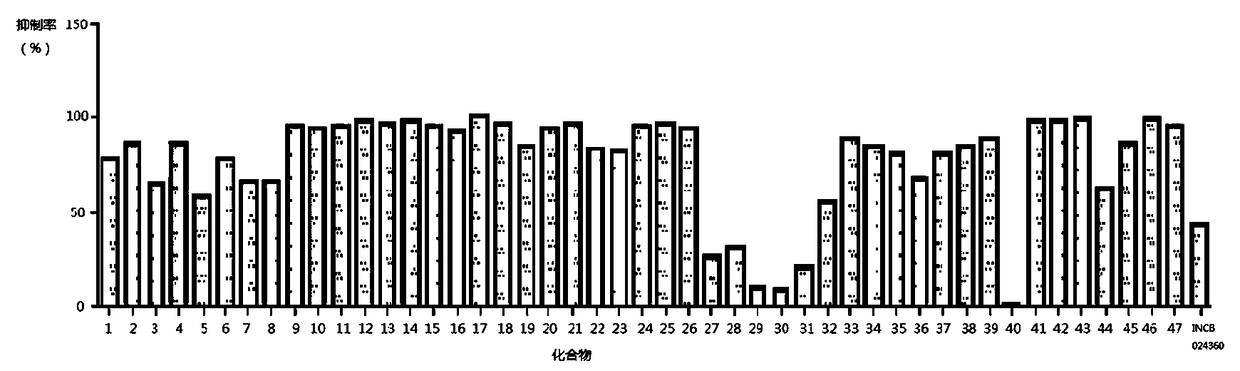
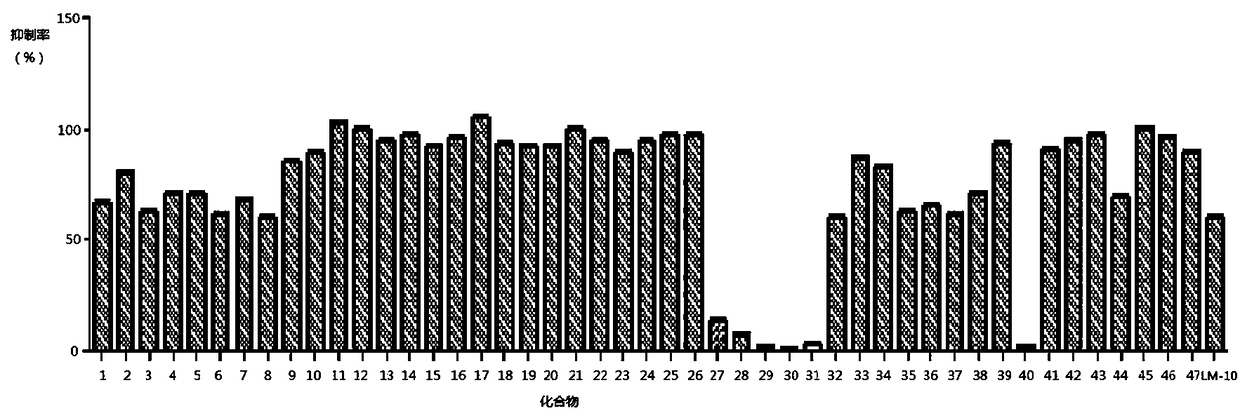
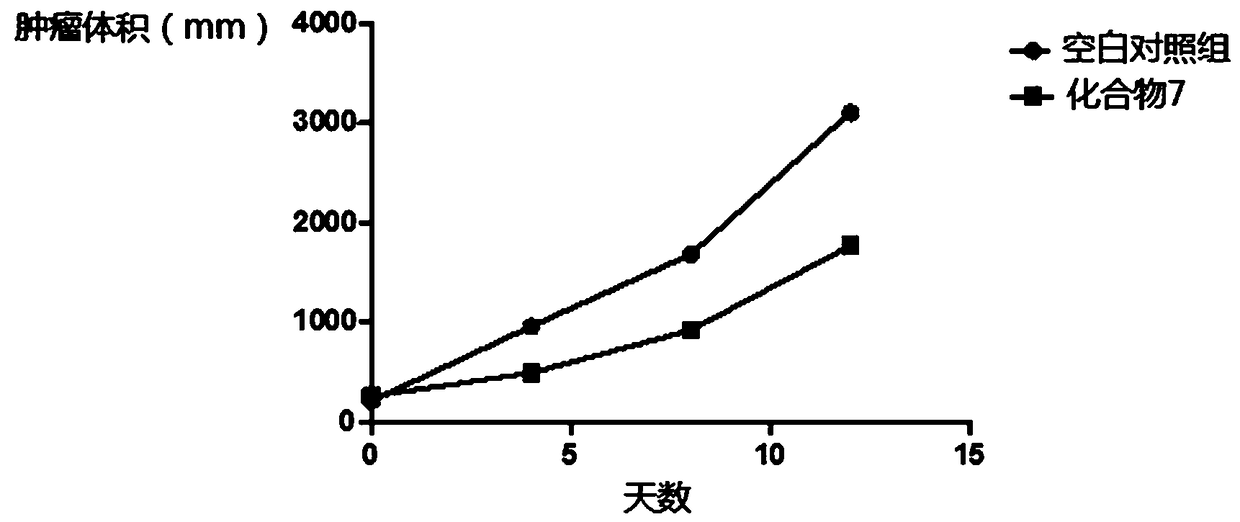
Image
Examples
Embodiment 1
[0049] Compound 1: 1-Phenyl-1H-naphtho[2,3-d][1,2,3]triazole-4,9-dione
[0050] Use raw material A as (raw material A1), raw material B is (raw material B1).
[0051] The synthetic route is as follows:
[0052]
[0053] The specific synthesis method is:
[0054] (1) Add raw material A1 (1 g, 6.85 mmol) and strong base potassium tert-butoxide (3.84 g, 34.25 mmol) into 12 mL of anhydrous tert-butanol solvent, then stir for 2 h at room temperature under an oxygen atmosphere of 1 atm. After the reaction, dilute hydrochloric acid was added to the reaction system to adjust the pH to 1-2, a yellow solid precipitated in the reaction system, and the intermediate I was obtained by suction filtration 1 , the reaction yield was 50%. Intermediate I 1 It can be directly used in the next reaction without further purification.
[0055] (2) Add raw material B1 (1g, 10.75mmol) and 20mL of acetonitrile into a 100mL round bottom flask and stir to dissolve at 0°C, then add t-BuONO (2.9...
Embodiment 2
[0060] In this embodiment, on the basis of the above-mentioned compounds, the raw material B was replaced, as follows:
[0061] Compound 2:
[0062] Use raw material A as (raw material A1), raw material B is (raw material B2)
[0063] The synthetic route is as follows:
[0064]
[0065] The specific preparation method is the same as the above-mentioned embodiment, and will not be repeated here.
[0066] The yield of compound 2 was 88%.
[0067] That 1 H NMR data are as follows:
[0068] 1 H NMR (400MHz, DMSO-d 6 )δ8.25(dd, J=7.3,1.6Hz,1H),8.13(dd,J=7.3,1.7Hz,1H),8.01–7.92(m,2H),7.92–7.85(m,2H),7.54 (t,J=8.8Hz,2H).ESI-MS m / z:296.08[M+H] + .
Embodiment 3
[0070]In this embodiment, on the basis of the above-mentioned compounds, the raw material B was replaced, as follows:
[0071] Compound 3:
[0072] Use raw material A as (raw material A1), raw material B is (Raw material B3)
[0073] The synthetic route is as follows:
[0074]
[0075] The specific preparation method is the same as the above-mentioned embodiment, and will not be repeated here.
[0076] The yield of compound 3 was 86%.
[0077] That 1 H NMR data are as follows:
[0078] 1 H NMR (400MHz, DMSO-d 6 )δ8.25(dd, J=7.3,1.7Hz,1H),8.15(dd,J=7.2,1.7Hz,1H),8.03–7.93(m,3H),7.86–7.76(m,2H),7.73 (dd,J=17.8,9.9Hz,1H).ESI-MS m / z:312.05[M+H]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com