Substituted cyclohexenones
A cyclic, alkenyl technology, applied in the field of preparation of optically active cyclohexenone derivatives
Active Publication Date: 2010-12-29
FIRMENICH SA
View PDF3 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, as will be shown further below, the methods described in the examples are not effective when used in the matrix of the present invention
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
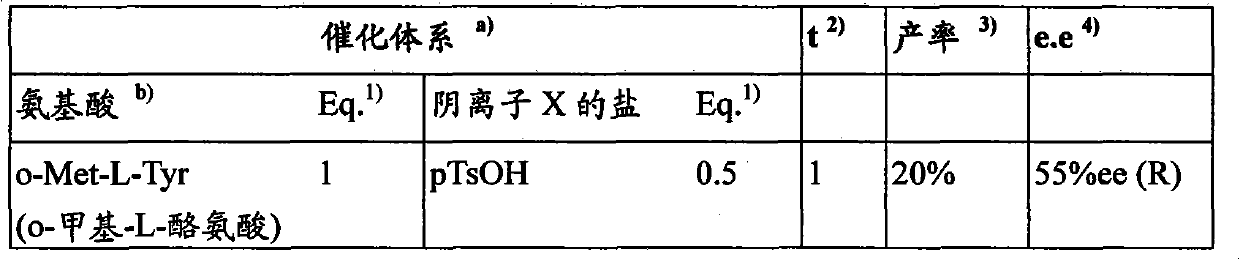
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more PUM
 Login to view more
Login to view more Abstract
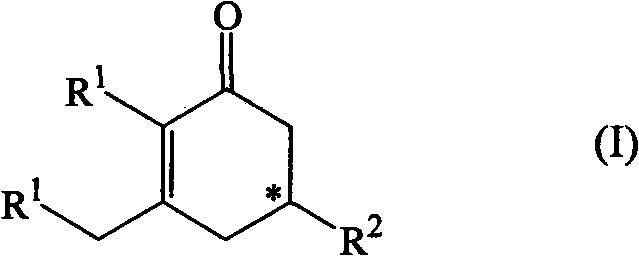
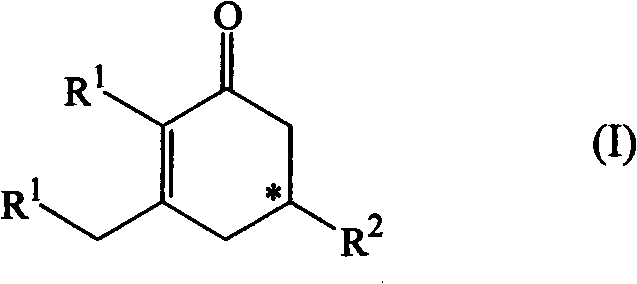
The present invention relates to a method of preparation of an optically active cyclohexenone derivative of Formula (I) and wherein R1 and R2 are organic residues.
Description
technical field The present invention relates to the field of organic synthesis, more particularly to a method for preparing optically active cyclohexenone derivatives of general formula (I), where R 1 and R 2 defined below. Background technique Optically active cyclohexenone derivatives are useful intermediates or building blocks for the synthesis of various more complex compounds, such as steroids or macrocyclic ketones, which are known as certain C 14 、C 15 or C 16 Useful musky fragrance like 3-methylcycloenone. Despite this fact, to the best of our knowledge, the prior art only reports very few methods for the preparation of optically active cyclohexenone derivatives from achiral diketones or aldol adducts. In patent applications WO 2005 / 077875 and WO 2007010483, the use of chiral amino alkoxides and the use of achiral diketones or achiral aldol adducts as starting materials are described. In another document (see C. Agami et al. in Bulletin de la Société Ch...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more Application Information
Patent Timeline
 Login to view more
Login to view more Patent Type & Authority Applications(China)
IPC IPC(8): C07C45/66C07C49/603C07C49/623
CPCC07C45/66C07C49/623C07C49/603
Inventor O·克诺普夫
Owner FIRMENICH SA
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap