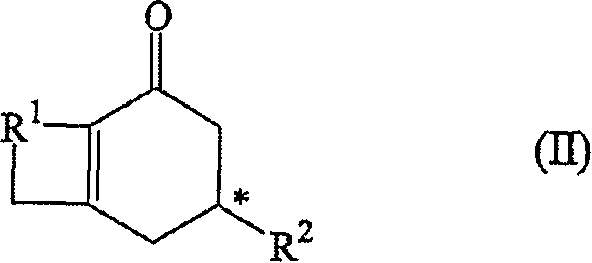
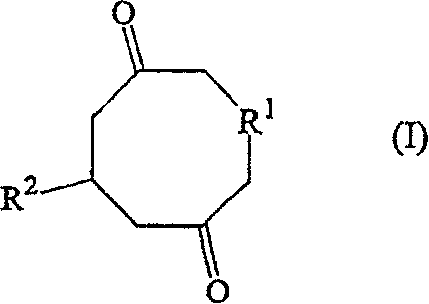
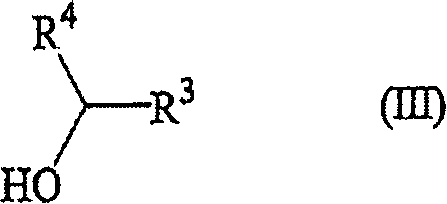A process for the preparation of optically active cyclohexenones
一种旋光性、非强制的技术,应用在有机化学方法、碳基化合物制备、化学仪器和方法等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0081] According to another embodiment of the present invention, the preferred additives are NaH, KH, 4 Ȧ type anhydrous zeolite, t BuONa or anhydrous KOH, NaOH, NaCl, Na 2 CO 3 、Na 2 SO 4 .
[0082] Said additives can be added to the reaction medium in a wide range of amounts, depending on the exact nature of the additive. Nevertheless, additions of more than three times the theoretical amount required to capture all the water theoretically produced do not provide any appreciable additional benefit.
[0083] The process of the present invention can be carried out in the presence or absence of a solvent, but in any case the reaction advantageously takes place under anhydrous conditions such as less than 0-5% w / w water. Those skilled in the art will appreciate that the presence of a solvent is only necessary if the starting diketone (I) is a solid compound under the reaction conditions.
[0084] However, according to a preferred embodiment of the invention, independent of...
Embodiment 1
[0089] Preparation of Optically Active 14-Methyl-bicyclo[9.4.0]pentadeca-1(11)-en-12-one
[0090]
[0091] a) General procedure:
[0092] Under an inert environment, add 126 mg 3-methyl-1,5-cyclopentadecanedione, 3 ml dry THF, optional 200 mg anhydrous 4 Å molecular sieves or 2 molar equivalents of NaH, and dissolve in Na alkoxides or K alkoxides 1-12 shown in Table 1 were dried in THF. The total amount of THF present was calculated to keep the starting diketone concentration between 0.1-0.4 mol / L at the beginning of the reaction.
[0093] The reaction mixture was stirred at room temperature, followed by GC analysis. To quench the reaction, the mixture was hydrolyzed with water or 2N aqueous HCl. After extracting the aqueous layer with diethyl ether, the organic layer was washed with MgSO 4 Dry and filter. The solvent was removed under vacuum and the residue was purified by flash chromatography or by bulb to bulb distillation to give the desired product, i.e. (S)-14-me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com