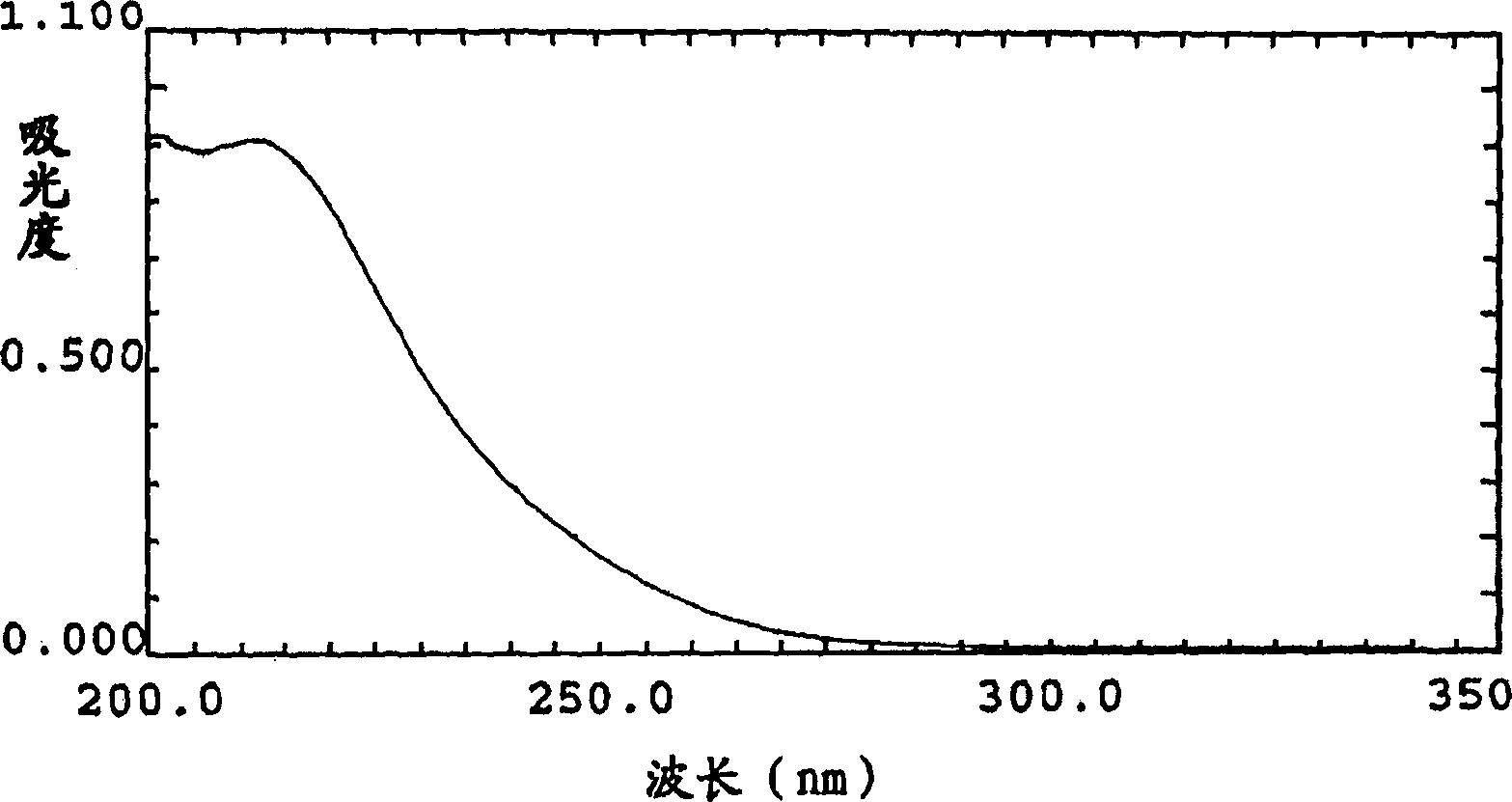
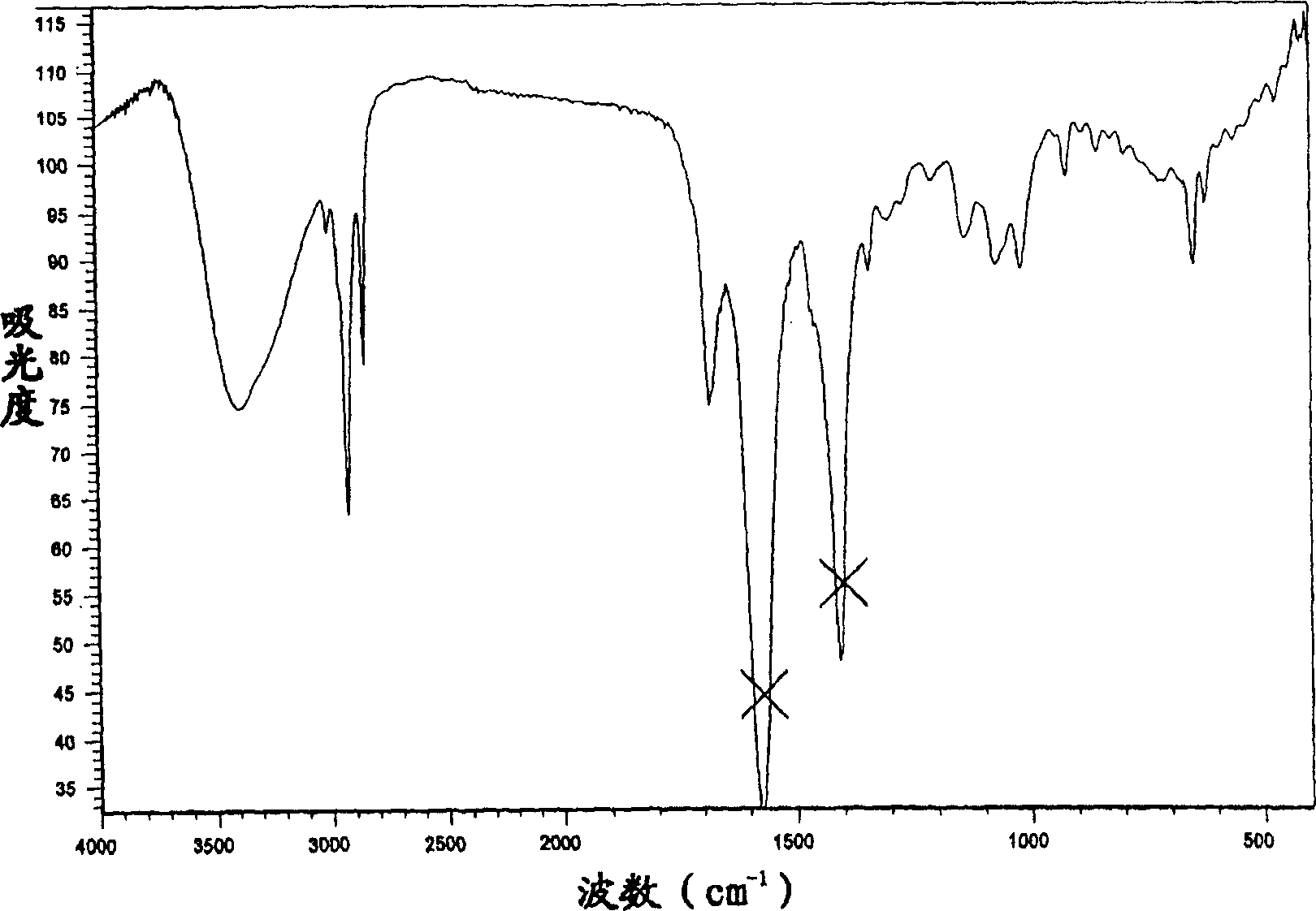
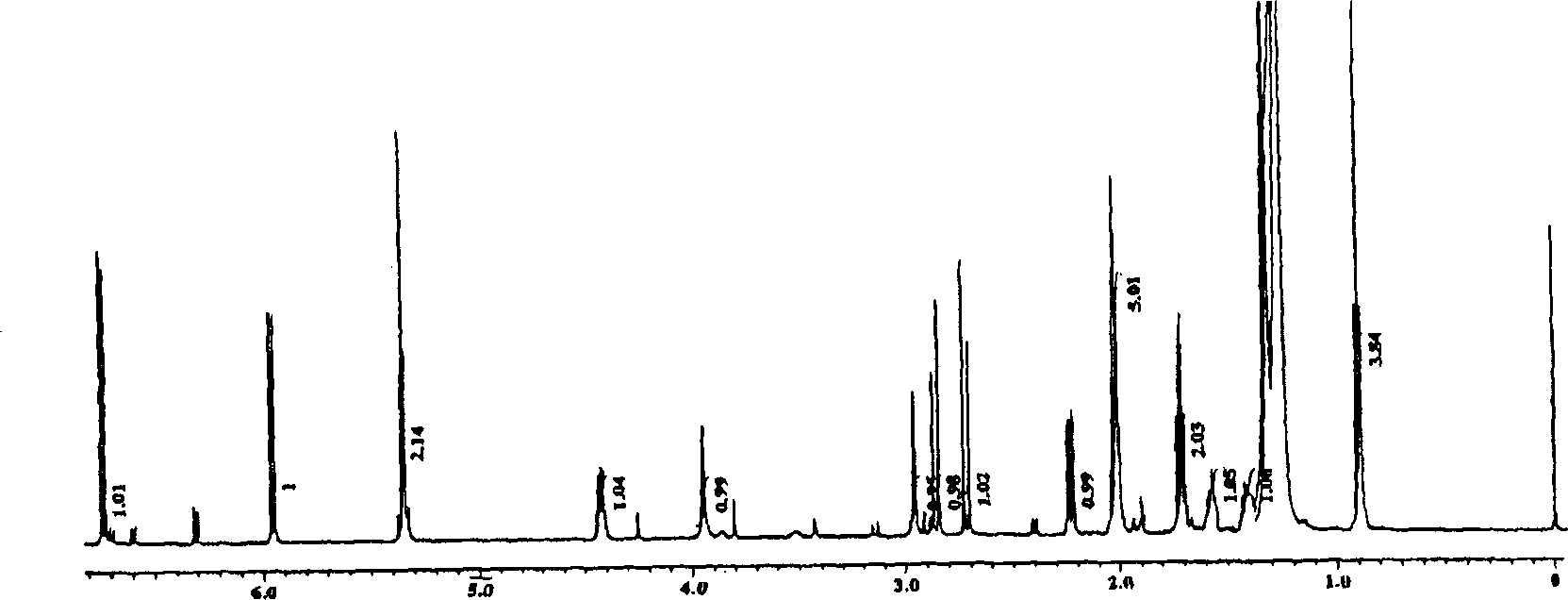
Cyclohexenone analog bicyclo (condensed ring) compound and its preparation method and uses
A compound and extract technology, applied in the field of cyclohexenone bicyclic new compounds, can solve the problems such as no research reports on anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The dry bark (3.2kg) of Choerospondias axillaries (Roxb.) Burtt et Hill was crushed and then extracted with 25 liters of ethanol at room temperature for 7 days each time, 4 times in total. The extracts were combined, concentrated and dried to obtain 750 g of ethanol extract. Suspend 750 g of the ethanol extract in 3 liters of water, extract with chloroform, and extract 4 times with 3 liters of chloroform each time. The chloroform extracts were combined and concentrated to dryness to obtain 60 g of extract.
[0055] The obtained extract (60 g) was dry-processed on a vacuum silica gel column (column bed 7.5 cm×18.5 cm), and gradient elution chromatography was performed using petroleum ether-acetone (100:1→2:1) solvent system as the eluent. During the elution process, the polarity of the elution solvent (V P :V A =100:1, 50:1, 10:1, 5:1, etc.). Merge fractions according to TLC detection and activity test results, at V P :V A = 5:1 eluted fraction yielded 10.8 g of ex...
Embodiment 2
[0078] Example 2. Biological activity test
[0079] Experimental samples and experimental methods
[0080] Preparation of the tested sample solution: take the pure compound II isolated and refined in the above Example 1, accurately weigh an appropriate amount, and prepare a solution with the required concentration with methanol for testing the activity.
[0081] Cell lines and cell culture: Mouse breast cancer tsFT210 cell line, human colorectal cancer HCT-15 cell line, human cervical cancer HeLa cell line, human breast cancer MCF-7 cell line, and human ovarian cancer A2780 cell line were used for activity testing.
[0082] Cells were subcultured in RPMI-1640 medium containing 10% FBS at 32°C (tsFT210 cells) or 37°C (HCT-15, HeLa, MCF-7 and A2780 cells) in an incubator filled with 5% carbon dioxide .
[0083] Cell proliferation inhibitory activity test method (Lissamine rhodamine B method or SRB method): human colon cancer HCT-15 cells in logarithmic growth phase, human cerv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com