Gastrin releasing peptide compounds
A composition, radionuclide technology, used in animal repellents, biomaterial analysis, peptide/protein composition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0502] The following examples are provided as examples of different methods that can be used to prepare the various compounds of the invention. In the various examples, there are compounds identified with a single bold capital letter (eg, A, B, C), which are related to the corresponding compounds with the same label in the drawings.
[0503] General experiment
[0504] A. Definition of other abbreviations used
[0505] The following common abbreviations are used throughout the manual:
[0506] 1,1-Dimethylethoxycarbonyl (Boc or Boc);
[0507] 9-fluorenylmethoxycarbonyl (Fmoc);
[0508] Allyloxycarbonyl (Aloc);
[0509] 1-Hydroxybenzotriazole (HOBt or HOBT);
[0510] N,N'-Diisopropylcarbodiimide (DIC);
[0511] N-Methylpyrrolidone (NMP);
[0512] Acetic anhydride (Ac 2 O);
[0513] (4,4-Dimethyl-2,6-dioxocyclohexylene-1-yl)-3-methylbutyl (iv-Dde);
[0514] Trifluoroacetic acid (TFA);
[0515] Reagent B (TFA: H 2 O:phenol:triisopropylsilane, 88:5:5:2);
[0516] Diisopropylethylamine (DIEA);
[051...
Embodiment I-
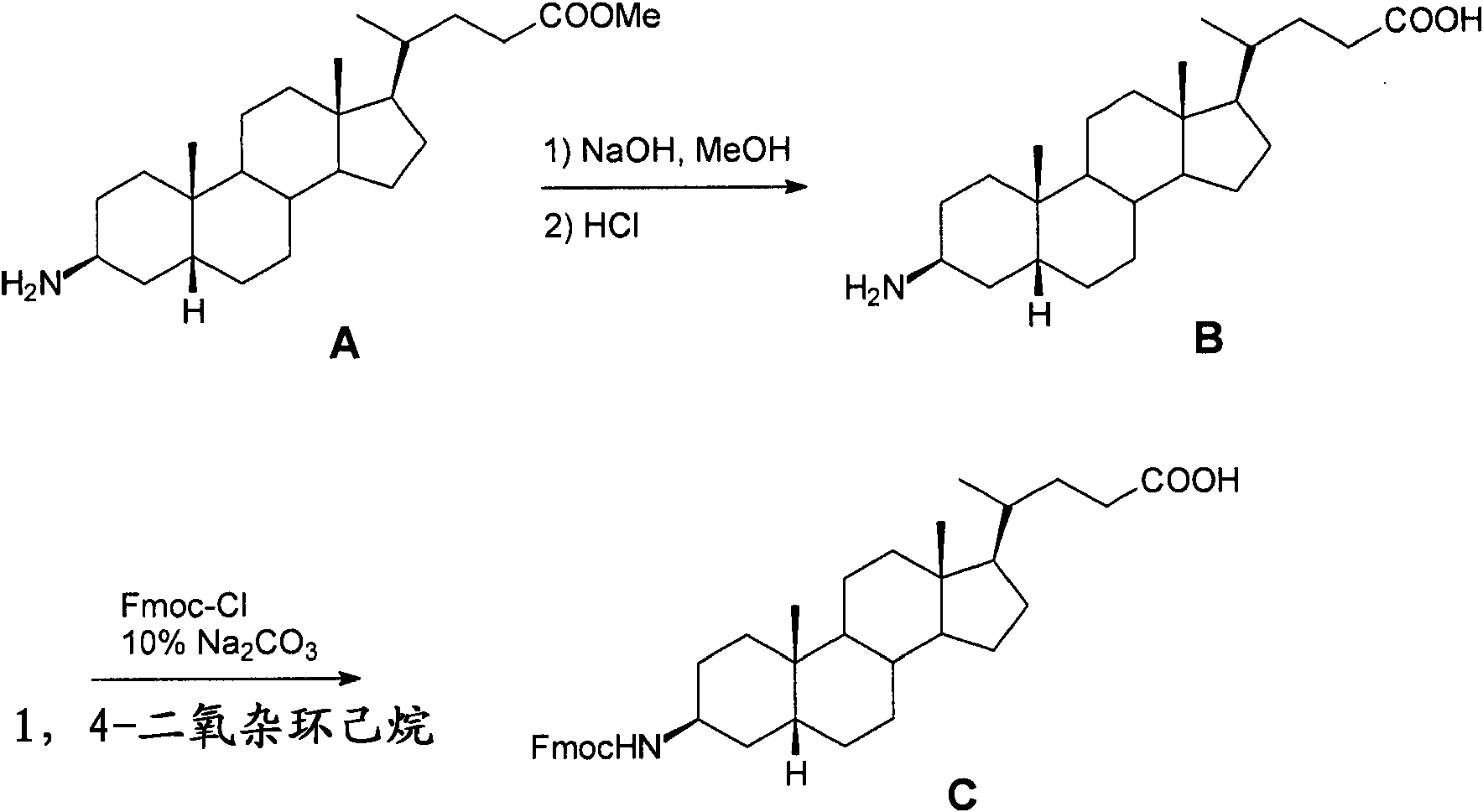
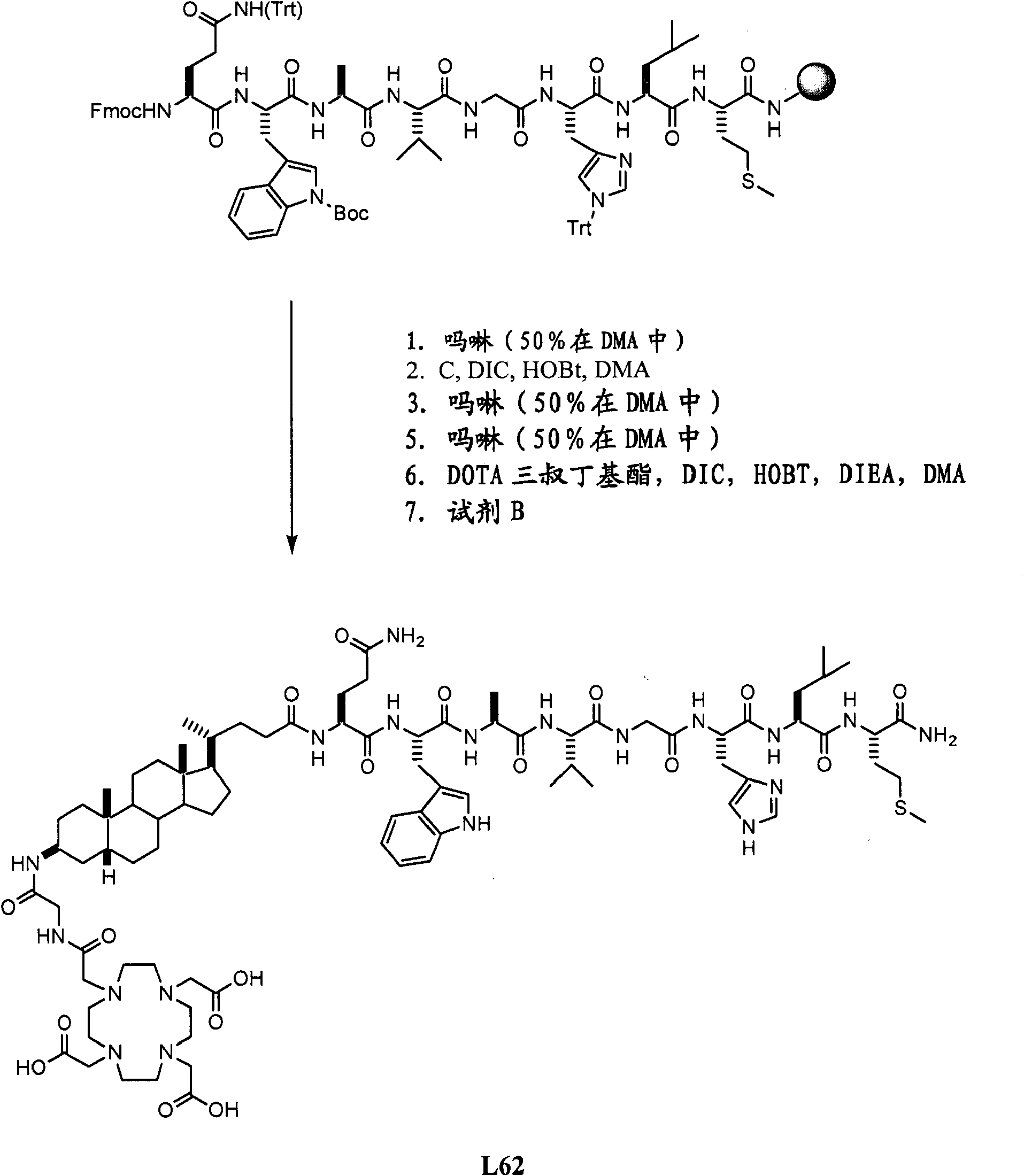
[0564] Example I- Figure 1A -B
[0565] Synthetic L62
[0566] Overview: such as Figure 1A As shown in -B, L62 is prepared with the following steps:
[0567] (3β,5β)-3-aminocholane-24-acid methyl ester A was hydrolyzed with NaOH to obtain the corresponding acid B, and then reacted with Fmoc-Cl to obtain intermediate product C. Use octapeptide Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH 2 (BBN[7-14][SEQ ID NO: 1]) functionalized Rink amide resin was reacted with C, Fmoc-glycine and DOTA tri-tert-butyl ester in sequence. After cleavage and deprotection with reagent B, the crude product was purified by preparative HPLC to obtain L62. Total yield: 2.5%. More details are provided below:
[0568] A. Rink amide resin functionalized with bombesin [7-14] (SEQ ID NO:1), (A)
[0569] In a solid-phase peptide synthesis container, mix Fmoc-amino acid (24mmol), N-hydroxybenzotriazole (HOBt) (3.67g; 24mmol) and N,N'-diisopropylcarbodiimide (DIC) ( 3.75mL; 24mmol) were added sequentially to Rink Amide No...
Embodiment II-
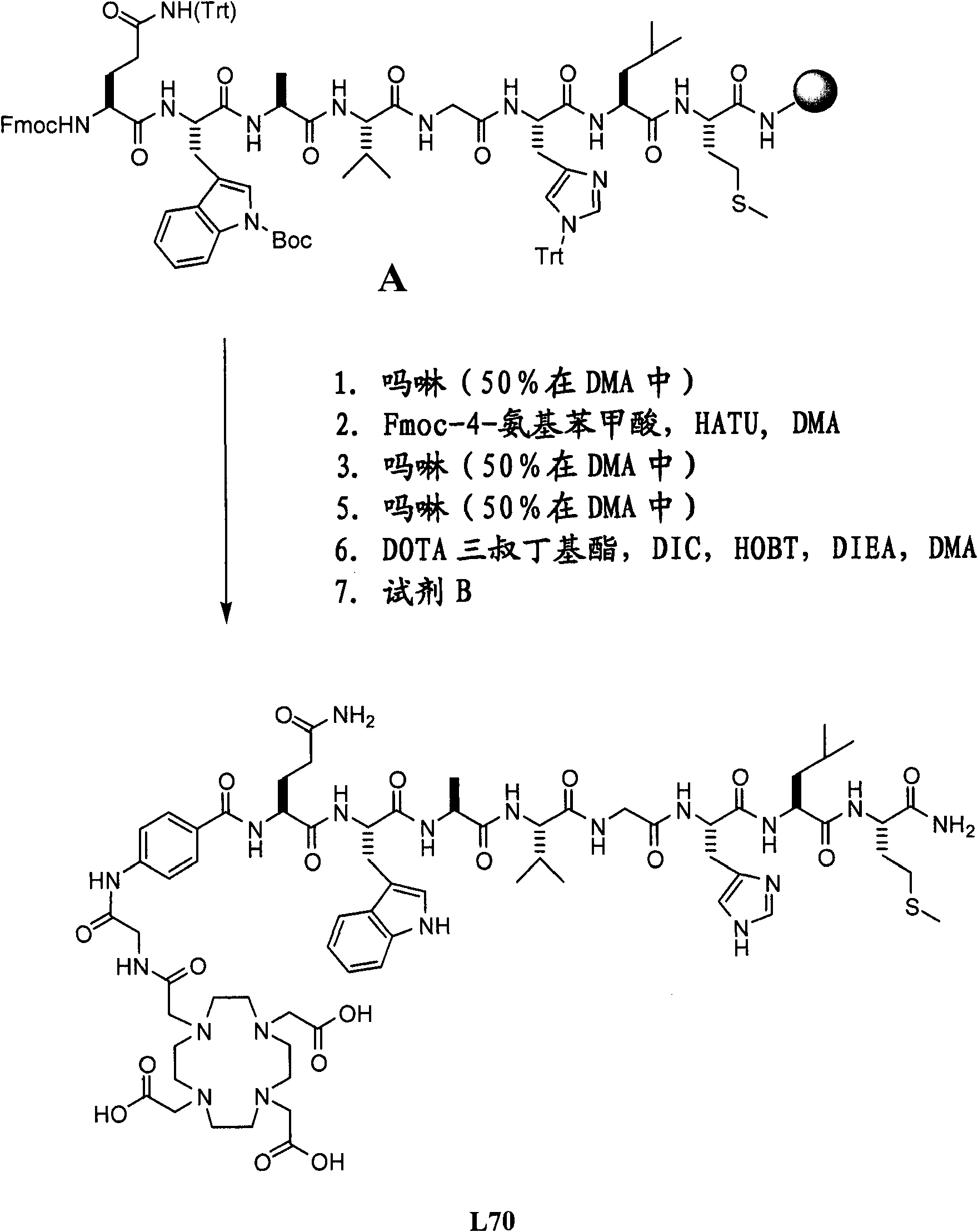
[0580] Example II- Figure 2A -F
[0581] Synthesize L70, L73, L74, L115 and L116
[0582] Overview: The product is obtained by the following method: The octapeptide Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH on Rink amide resin 2 (BBN[7-14][SEQ ID NO:1]) (with suitable side chain protection) was coupled with different linkers, and then functionalized with DOTA tri-tert-butyl ester. After cleavage and deprotection with reagent B, the final product was purified by preparative HPLC. The total yield is 3-9%.
[0583] A. Synthetic L70 ( Figure 2A ):
[0584] Resin A (0.5g; 0.3mmol) was shaken with 50% morpholine in DMA (7mL) in a solid phase peptide synthesis vessel for 10 minutes, the solution was emptied and fresh 50% in DMA (7mL) was added Morpholine. The suspension was stirred for 20 minutes, then the solution was emptied and the resin was washed with DMA (5×7 mL). Fmoc-4-aminobenzoic acid (0.43g; 1.2mmol), HOBT (0.18g; 1.2mmol), DIC (0.19mL; 1.2mmol) and DMA (7mL) were added to the resi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com