Detection method of compound danshen dripping pills
A technology of compound salvia miltiorrhiza and detection method, which is applied in the field of detection of compound salvia miltiorrhiza dripping pills, can solve problems affecting product production and quality assurance, simple quality control methods, and difficult control of product quality, achieving stable quality, good stability, curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
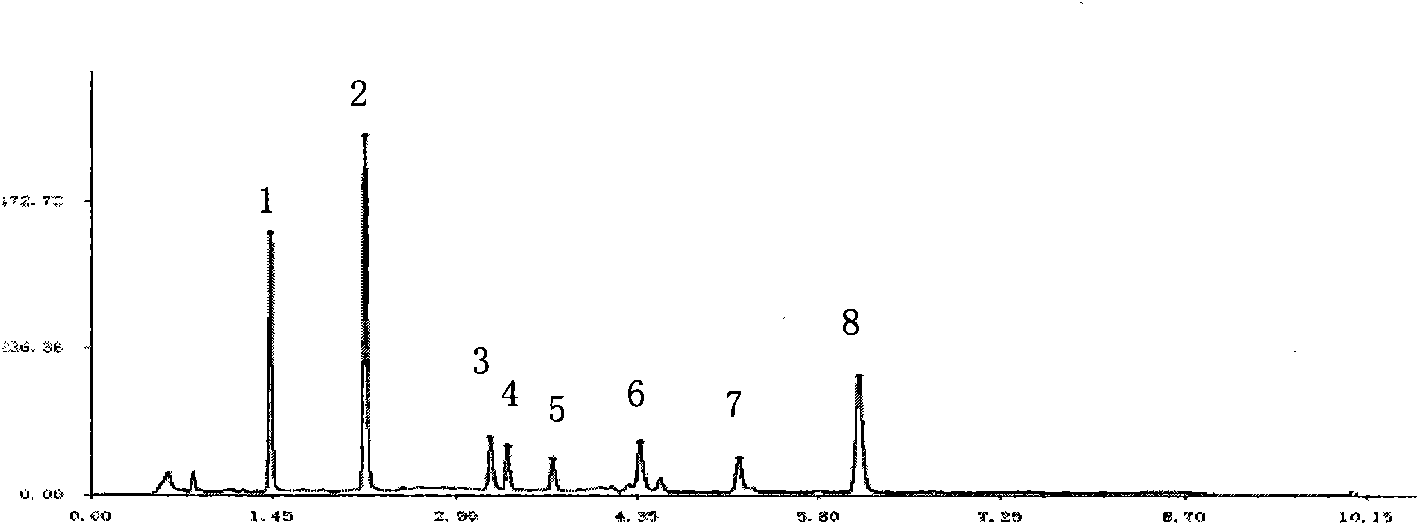
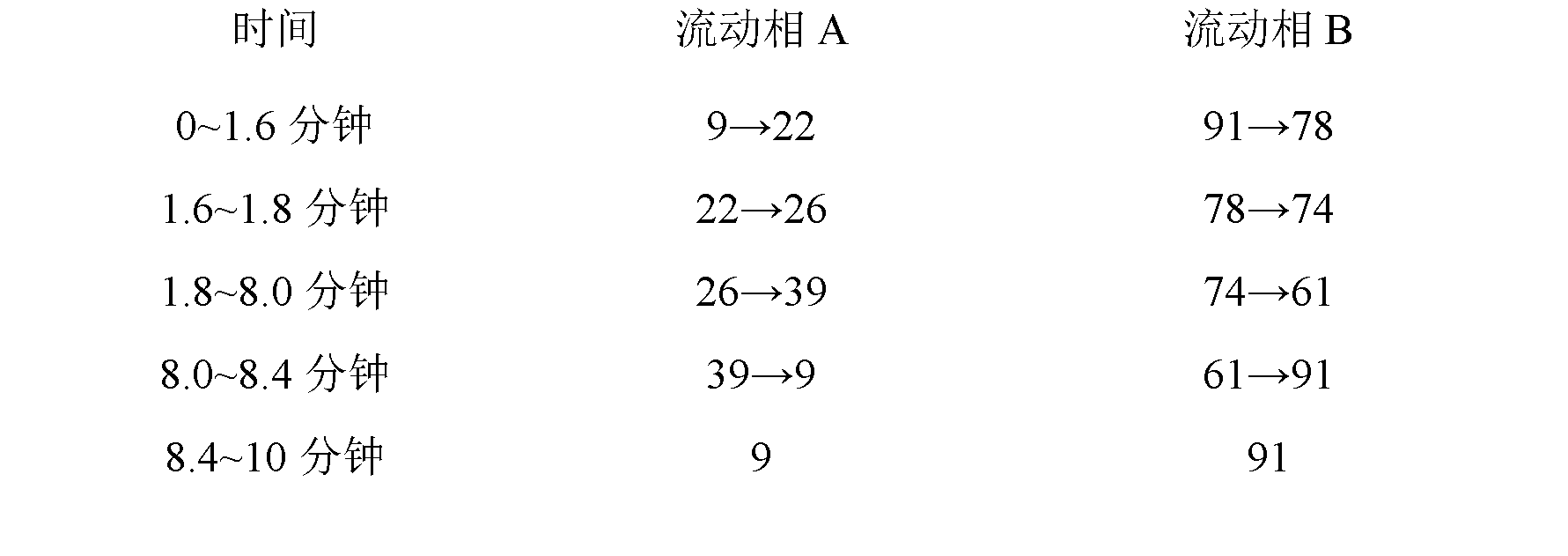
[0048] Establishment of Fingerprint Analysis Method for Compound Danshen Dripping Pills
[0049] Liquid chromatography: Waters ultra-high performance liquid chromatography (UPLC)
[0050] Agilent Rapid Resolution Liquid Chromatograph (RRLC)
[0051] Shimadzu Ultrafast Liquid Chromatography (UFLC)
[0052] The above three liquid chromatographs use sub-2μm liquid chromatography technology, which has the same theory and principle as ordinary HPLC. The different sub-2μm liquid chromatography covers small particle packing, low system volume and rapid detection methods, etc. The new technology increases the throughput, sensitivity and chromatographic peak capacity of the analysis, making it more sensitive, with a higher theoretical plate number, better separation effect, shorter peak time, and at the same time reducing the use of toxic solvents. Conducive to environmental protection. Selecting sub-2μm liquid chromatography technology to develop fingerprints and content determinat...
Embodiment 2
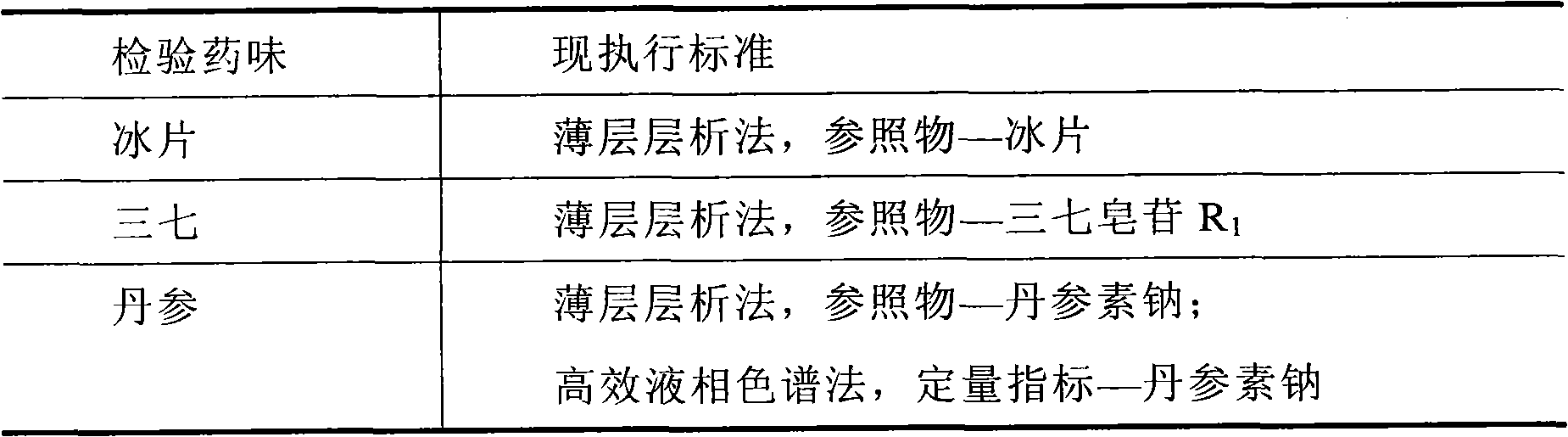
[0091] Using thin-layer chromatography, notoginseng was used as the control medicinal material to identify the notoginseng in the prescription.
[0092] The preparation of need testing solution: get this product 20 balls, put in centrifuge tube, add dilute ammonia solution (8ml → 100ml) 9ml, sonication makes to dissolve, centrifugation, get supernatant, pass through D101 type macroporous adsorption resin column ( The inner diameter is 0.7cm, and the column height is 5cm), elute with 15ml of water, discard the water eluate, and then elute with methanol, discard about 0.4ml of the initial eluent, collect about 5ml of the subsequent eluent, and concentrate to about 2ml, as the test solution.
[0093] Preparation of the reference medicinal material solution: Take 0.5 g of the reference medicinal material of Panax notoginseng (batch number: 120941-200405), put it in a centrifuge tube, add 9 ml of dilute ammonia solution (8ml→100ml), sonicate for 15 minutes, centrifuge, take the sup...
Embodiment 3
[0102] Determination of Danshensu content
[0103] The content determination method of Danshensu:
[0104] The identification method is the same as that of the fingerprint, with Danshensu as the quantitative index, and the sub-2μm liquid chromatography technique is used to determine the content of Danshensu in the prescription.
[0105] Instruments and reagents
[0106] Instrument: Waters ACQUITY ultra-high performance liquid chromatography (UPLC)
[0107] Reference substance: Danshensu Sodium Reference substance: China Institute for the Control of Pharmaceutical and Biological Products, batch number: 110855-200608
[0108] Sample: Compound Danshen dripping pills were provided by Tianjin Tasly Pharmaceutical Co., Ltd. (batch numbers 20080705, 090201, 090202, 090203, 090204, 090205, 090206, 090207, 090208, 090209, 090210).
[0109] Acetonitrile: (chromatographically pure) German Merck company, batch number: I409030804
[0110] Phosphoric acid: (analytical pure) Tianjin Seco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com