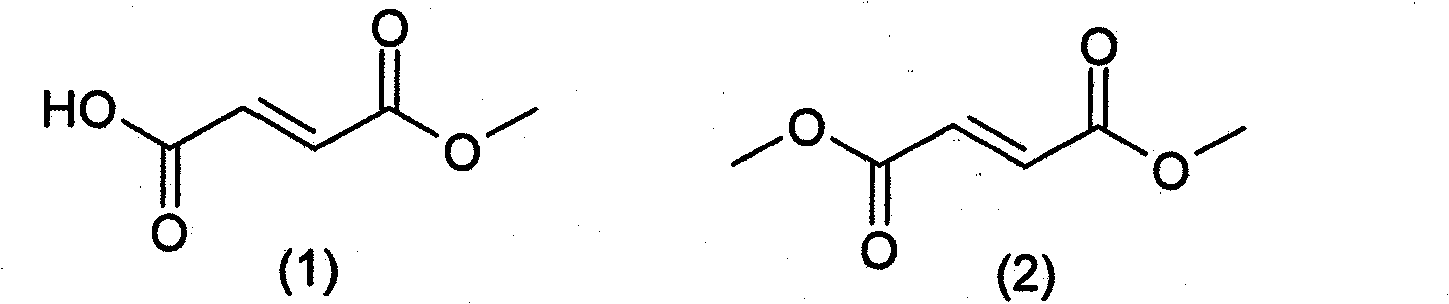
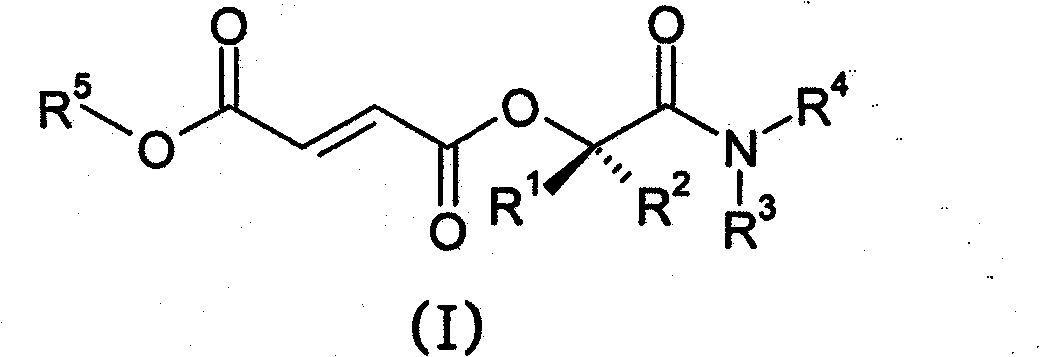
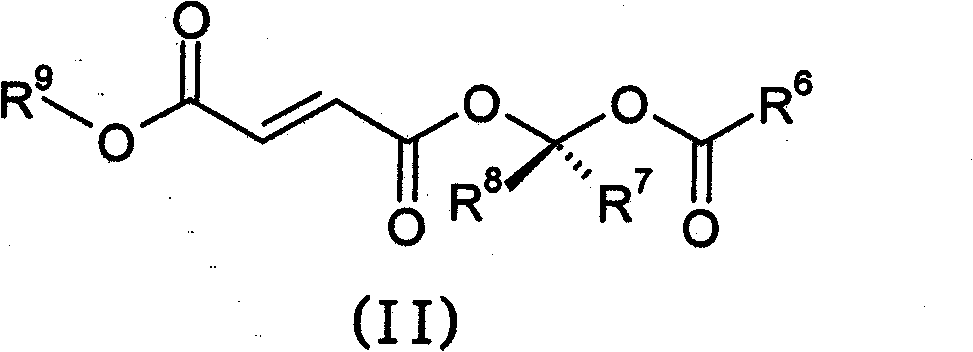
Prodrugs of methyl hydrogen fumarate, pharmaceutical compositions thereof, and methods of use
A kind of compound, the technology of di-ester, is applied in the prodrug of methyl hydrogen fumarate, its pharmaceutical composition, and its use field, can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0480] (N,N-Diethylcarbamoyl)methylmethyl(2E)but-2-ene-1,4-dioate (1)
[0481]
[0482] According to General Procedure A, methyl hydrogen fumarate (MHF) (0.39 g, 3.00 mmol) dissolved in NMP was mixed with 2-chloro-N,N-diethylacetamide (0.44 g, 3.00 mmol) at about 55 °C ) in CsHCO 3 (0.69 g, 3.60 mmol) gave 0.37 g ( 51% yield) of the title compound (1). M.p.: 53-56°C. 1 H NMR (CDCl 3 , 400MHz): δ6.99-6.90(m, 2H), 4.83(s, 2H), 3.80(s, 3H), 3.39(q, J=7.2Hz, 2H), 3.26(q, J=7.2Hz, 2H), 1.24(t, J=7.2Hz, 3H), 1.14(t, J=7.2Hz, 3H). MS(ESI): m / z 244.13(M+H) + .
Embodiment 2
[0484] Methyl[N-benzylcarbamoyl]methyl(2E)but-2-ene-1,4-dioate (2)
[0485]
[0486] According to General Procedure A, methyl hydrogen fumarate (MHF) (0.50 g, 3.85 mmol) dissolved in NMP was mixed with N-benzylchloroacetamide (0.84 g, 4.61 mmol) in CsHCO at about 55 °C. 3 Reaction in the presence of (0.89 g, 4.61 mmol) afforded 0.56 g (53% yield) of the title compound (2) as a white solid after purification by mass-directed preparative HPLC and lyophilization. 1 H NMR (CDCl 3 , 400MHz): δ7.36-7.26(m, 5H), 6.94-6.88(m, 2H), 6.19(br s, 1H), 4.73(s, 2H), 4.51(d, J=5.6Hz, 2H) , 3.81(s, 3H). MS(ESI): m / z 278.04(M+H) + .
Embodiment 3
[0488] Methyl 2-morpholin-4-yl-2-oxoethyl(2E)but-2-ene-1,4-dioate (3)
[0489]
[0490] According to General Procedure A, methyl hydrogen fumarate (MHF) (0.50 g, 3.84 mmol) dissolved in NMP was mixed with 4-(chloroacetyl)morpholine (0.75 g, 4.61 mmol) in CsHCO at about 55 °C. 3 Reaction in the presence of (0.89 g, 4.61 mmol) afforded 0.34 g (35% yield) of the title compound (3) as a white solid after purification by mass-directed preparative HPLC and lyophilization. M.p.: 124-126°C; 1 H NMR (CDCl 3 , 400MHz): δ6.97-6.91(m, 2H), 4.84(s, 2H), 3.82(s, 3H), 3.72-3.70(m, 4H), 3.64-3.62(m, 2H), 3.46-3.41 (m, 2H). MS(ESI): m / z 258.04(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com