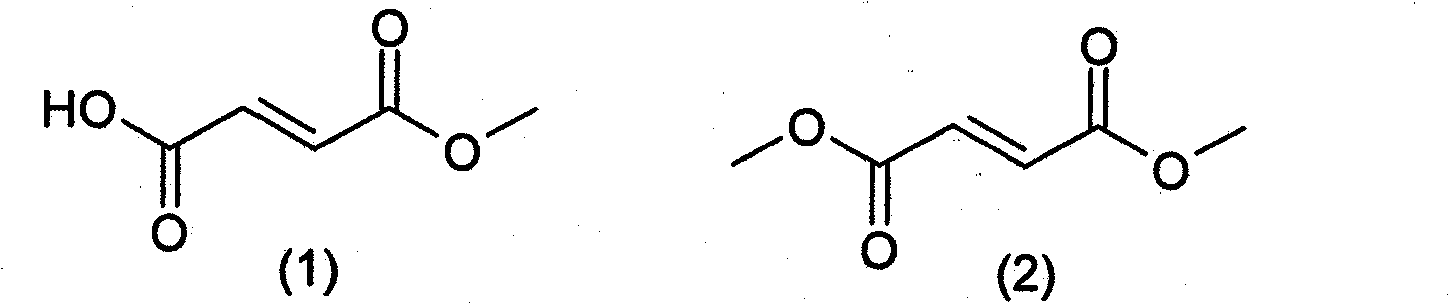
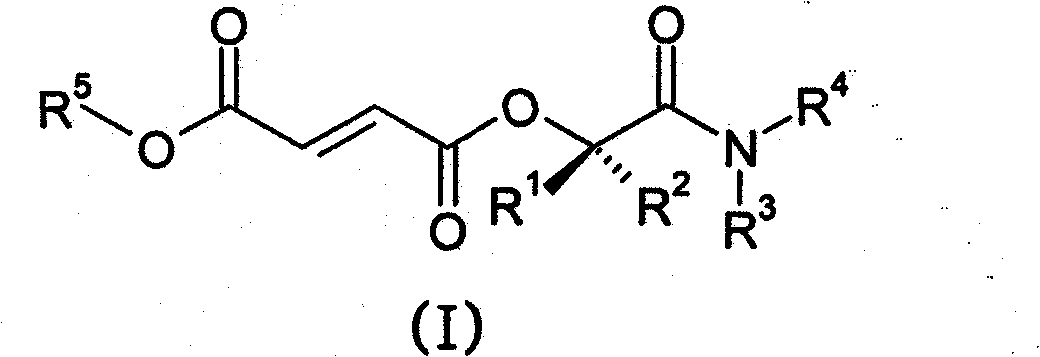
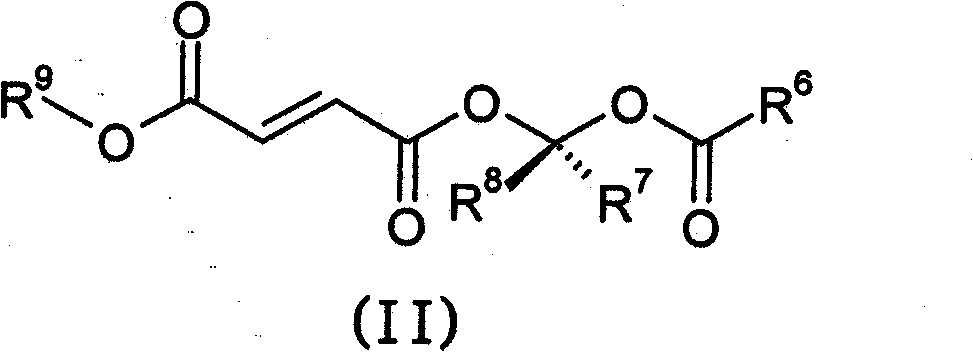
Prodrugs of methyl hydrogen fumarate, pharmaceutical compositions thereof, and methods of use thereof
A kind of composition, the technology of medicine, is applied in the prodrug of methyl hydrogen fumarate, its pharmaceutical composition, and its use field, can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0480] (N,N-Diethylcarbamoyl)methylmethyl(2E)but-2-ene-1,4-dioate (1)
[0481]
[0482] According to General Procedure A, methyl hydrogen fumarate (MHF) (0.39 g, 3.00 mmol) dissolved in NMP was mixed with 2-chloro-N,N-diethylacetamide (0.44 g, 3.00 mmol) at about 55 °C ) in CsHCO 3 (0.69 g, 3.60 mmol) gave 0.37 g ( 51% yield) of the title compound (1). M.p.: 53-56°C. 1 H NMR (CDCl 3 , 400MHz): δ6.99-6.90(m, 2H), 4.83(s, 2H), 3.80(s, 3H), 3.39(q, J=7.2Hz, 2H), 3.26(q, J=7.2Hz, 2H), 1.24(t, J=7.2Hz, 3H), 1.14(t, J=7.2Hz, 3H). MS(ESI):m / z244.13(M+H) + .
Embodiment 2
[0484] Methyl[N-benzylcarbamoyl]methyl(2E)but-2-ene-1,4-dioate (2)
[0485]
[0486] According to General Procedure A, methyl hydrogen fumarate (MHF) (0.50 g, 3.85 mmol) dissolved in NMP was mixed with N-benzylchloroacetamide (0.84 g, 4.61 mmol) in CsHCO at about 55 °C. 3 Reaction in the presence of (0.89 g, 4.61 mmol) afforded 0.56 g (53% yield) of the title compound (2) as a white solid after purification by mass-directed preparative HPLC and lyophilization. 1 H NMR (CDCl 3 , 400MHz): δ7.36-7.26(m, 5H), 6.94-6.88(m, 2H), 6.19(br s, 1H), 4.73(s, 2H), 4.51(d, J=5.6Hz, 2H) , 3.81(s, 3H). MS(ESI): m / z 278.04(M+H) + .
Embodiment 3
[0488] Methyl 2-morpholin-4-yl-2-oxoethyl(2E)but-2-ene-1,4-dioate (3)
[0489]
[0490] According to General Procedure A, methyl hydrogen fumarate (MHF) (0.50 g, 3.84 mmol) dissolved in NMP was mixed with 4-(chloroacetyl)morpholine (0.75 g, 4.61 mmol) in CsHCO at about 55 °C. 3 Reaction in the presence of (0.89 g, 4.61 mmol) afforded 0.34 g (35% yield) of the title compound (3) as a white solid after purification by mass-directed preparative HPLC and lyophilization. M.p.: 124-126°C; 1 H NMR (CDCl 3 , 400MHz): δ6.97-6.91(m, 2H), 4.84(s, 2H), 3.82(s, 3H), 3.72-3.70(m, 4H), 3.64-3.62(m, 2H), 3.46-3.41 (m, 2H). MS(ESI): m / z 258.04(M+H) + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com