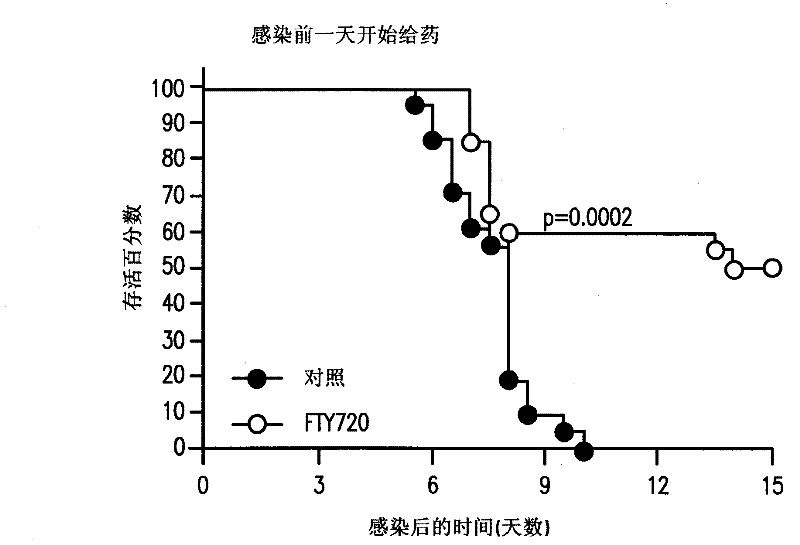
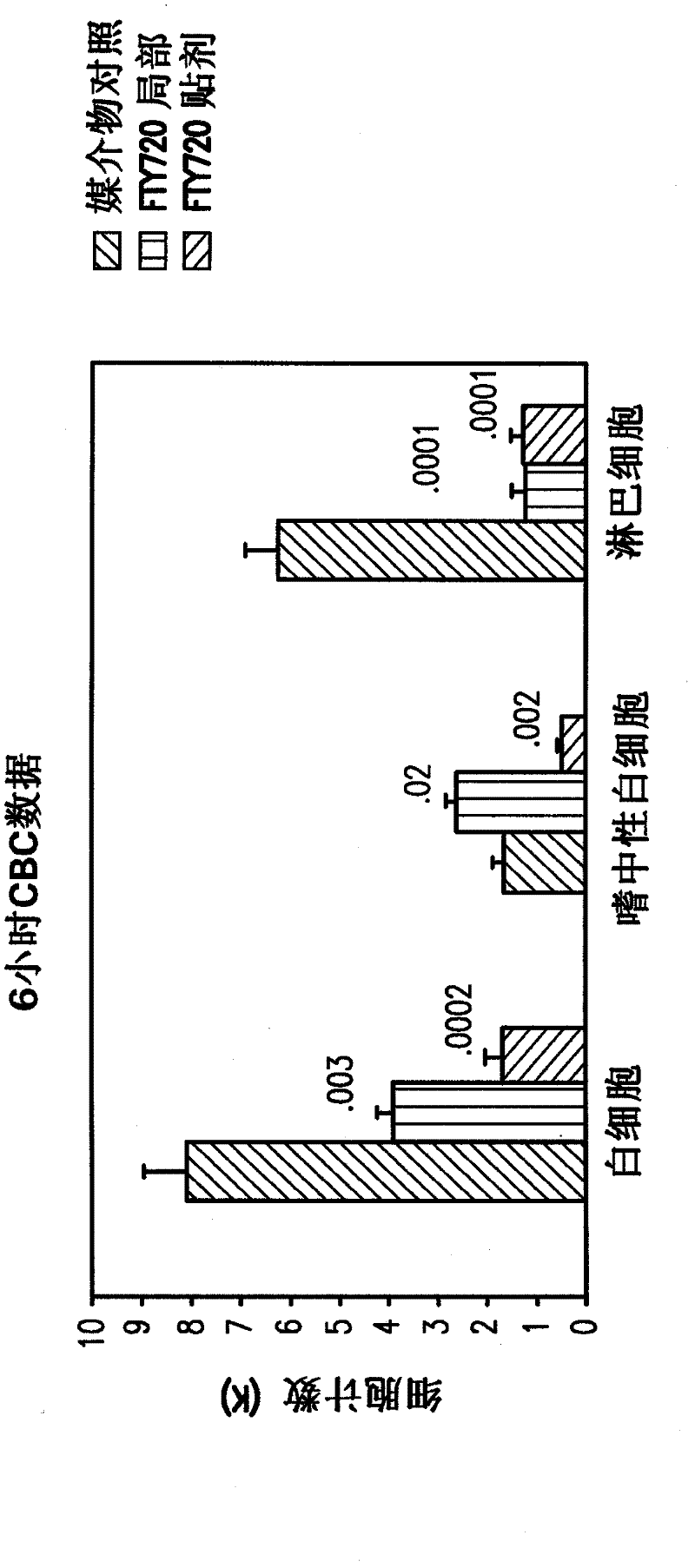
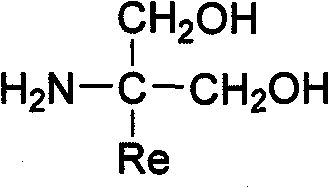
S1P receptor agonists for the treatement of cerebral malaria
A technology of receptor agonists and uses, applied in the direction of antibodies, anti-toxins, anti-inflammatory agents, etc., can solve problems such as no future for treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0114] Aspects of the invention can be understood from the following examples, which are not intended to limit the scope of the invention.
[0115] Measuring S1P receptor binding affinity
[0116] The binding affinity of an S1P receptor agonist for an individual human S1P receptor can be determined using well known assays. For example, the human S1P receptor S1P can be used 1 , S1P 2 , S1P 3 , S1P 4 and S1P 5 , by quantifying the induction of GTP [γ- 35 S] to test compounds. A suitable assay technique is SPA (scintillation proximity based assay). Briefly, compounds dissolved in DMSO were serially diluted and prepared in 50 mM Hepes, 100 mM NaCl, 10 mM MgCl 2 , 10μM GDP, 0.1% defatted BSA and 0.2nM GTP[γ- 35 S1P receptor expressing membrane protein (10-20 μg / well) immobilized with SPA-beads (Amersham-Pharmacia) in the presence of S] 1200 Ci / mmol). Unbound GTP[γ- 35 S]. Quantification of membrane-bound GTP [γ- 35 S] Fluorescence induced by SPA beads. Calculate EC u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com