Coxsackie virus A16 (CA16) detection testing strip
A Coxsackie virus and detection test paper technology, applied to measuring devices, instruments, scientific instruments, etc., to achieve the effects of simple operation, clear and easy to distinguish results, and easy promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of anti-Coxsackie virus A16 type VP1 protein monoclonal antibody
[0030] (1) Immunized mice
[0031] After the prepared genetically engineered antigen was taken out from the -20 low-temperature refrigerator and dissolved, it was subcutaneously injected into the back of BALB / C mice (0.2 mg / mouse) with an interval of 10 days. Three days before fusion, mice were challenged with 0.1 mg of antigen in the peritoneal cavity. The immune effect was detected by ELISA method.
[0032] (2) Myeloma cells
[0033] SP2 / 0 myeloma cells: purchased from the Institute of Microbiology and Epidemiology, Academy of Military Medical Sciences. Resuscitate the SP2 / 0 cells stored in the liquid nitrogen tank, and culture them in DMEM medium containing 10% calf serum for 48-72 hours. Number split, ready to fuse.
[0034] (3) cell fusion
[0035] Prepared SP2 / 0 cells and splenic lymphocytes of immunized BALB / C mice were prepared in 2×10 7 / ml and 1×10 8 / ml. Take...
Embodiment 2
[0042] Embodiment 2: Assay of anti-Coxsackie virus A16 type VP1 protein monoclonal antibody
[0043] (1) Monoclonal antibody ascites preparation:
[0044] Mice: SPF grade mice, no rodent virus contamination after inspection, if the animals are found to be unhealthy, bitten or infected during the ascites production process, they should be discarded.
[0045] Expanded culture of cell lines: take one tube of the production batch of cells to resuscitate, add nutrient solution to expand the culture, one production cell is only used once, and will not be frozen.
[0046]Inoculation of hybridoma cell lines: preparation of ascites should be carried out under sterile conditions, before injection of hybridoma cells, each mouse was intraperitoneally injected with liquid paraffin 0.5ml. One week later, each mouse was intraperitoneally injected with hybridoma cells 1-3×10 6 / 0.2ml.
[0047] Ascites collection: 7-10 days after the injection of the cell line, or the ascites was collected ...
Embodiment 3
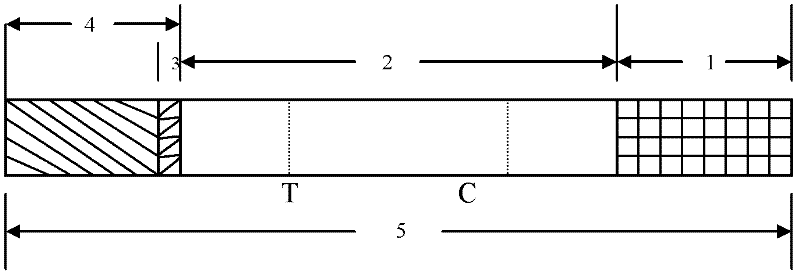
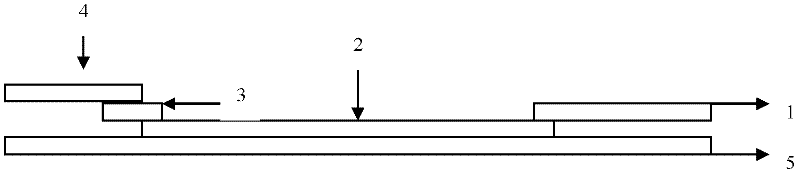
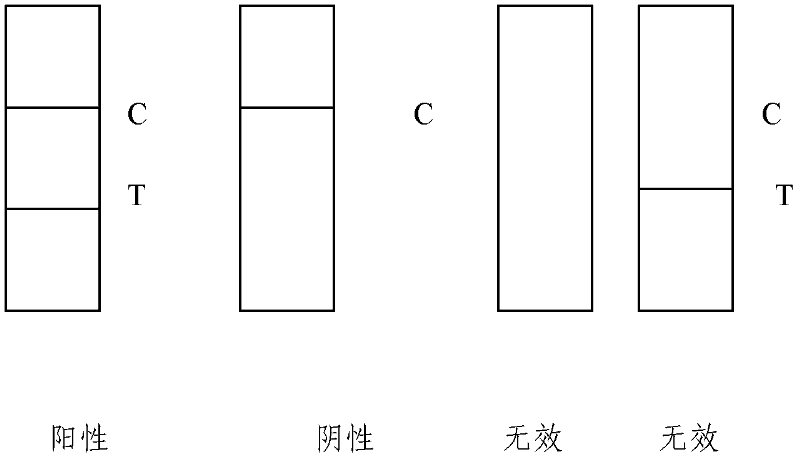
[0056] Embodiment 3: Coxsackie virus A16 type (CA16) detection test strip (colloidal gold) (see Fig. 1)
[0057] (1) Preparation of colloidal gold-VP1 monoclonal antibody conjugate:
[0058] It was determined by experiments that the optimum binding pH value of the anti-VP1 monoclonal antibody colloidal label was 8.0, and the ratio of colloidal gold and antibody was 40 μg / ml colloidal gold. After the labeled colloidal gold is treated with a stabilizer, the colloidal gold-antibody conjugate solution is taken in an amount of 65 μl per square centimeter, evenly adsorbed on glass fibers, freeze-dried, and stored in a dry environment.
[0059] (2) Coating antigen on nitrocellulose membrane:
[0060] Dilute another monoclonal antibody of VP1 that recognizes different antigenic sites (please confirm) with 0.01MPBS to 1.5mg / ml. Anti-mouse IgG polyclonal antibody was diluted to 2mg / ml with 0.01MPBS. Spray the two on the nitrocellulose membrane at a speed of 1 μl / cm with a film sprayi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap