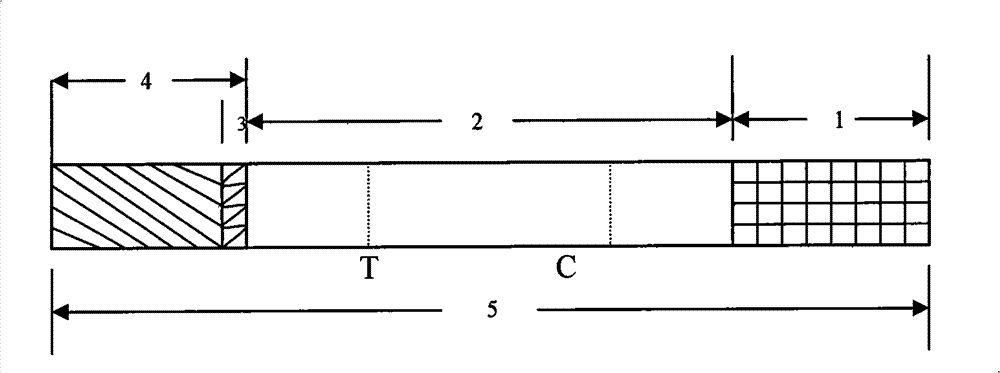
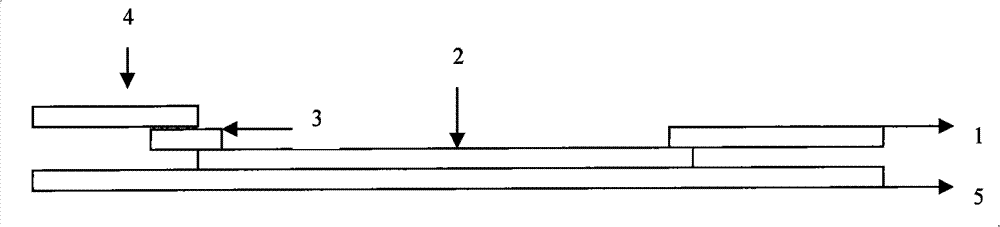
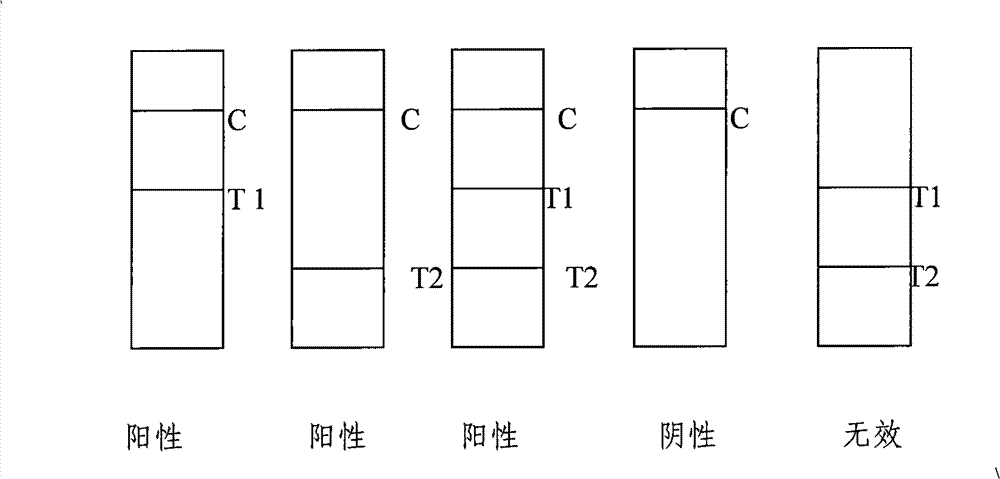
Test paper strip for detecting clostridium difficile toxin A and toxin B colloidal gold, method for making same
A Clostridium difficile and detection test paper technology, which is applied in the field of Clostridium difficile A and B toxin colloidal gold detection test strips, can solve the problems of cumbersome operation and long time, and achieve simple operation, clear and easy to distinguish results, and easy promotion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of Genetic Engineering Antigen of Clostridium difficile A Toxin
[0040] (1) Obtaining the target gene
[0041] According to the target gene fragment sequence (GenBank accession number is NC009012) and the characteristics of the pGEX-4T-1 (Pharmacia) expression vector, design primers containing restriction enzymes EcoR1 and Xho1 restriction sites at both ends:
[0042]5'TCCGAATTGGGCCTCAACTGGTTATACAAGT 3'
[0043] 5'GTGCTCGAGAGGGGCTTTTACTCCATCAAC3'
[0044] Then, the target gene fragment was amplified from the genome, and the amplification conditions were: denaturation at 95°C for 5 minutes; 35 cycles at 95°C for 1 minute, 1 minute at 49.8°C, and 1 minute at 70°C; finally, extension at 70°C for 10 minutes.
[0045] (2) Cloning of the target gene and screening of positive recombinants
[0046] After electrophoresis, the PCR amplified product was recovered by gel cutting, ligated with the PMD-18T cloning vector overnight at 16°C, transformed into D...
Embodiment 2
[0055] Example 2: Preparation of Clostridium difficile B toxin genetically engineered antigen
[0056] (1) Amplification of the target gene
[0057] Refer to the Cd VPI10463 gene sequence included in GenBank, design primers, and add a suitable restriction endonuclease cutting site at its 5' end:
[0058] Upstream primer: 5'TCCGAATTCGCTTATGTCAACTAGTGAA 3'
[0059] Downstream primer: 5'GCACTCGAGTTCACTA ATCACT AATTC 3'
[0060] The total reaction system is 50ul: upstream primer (20pmol / L) 1ul, downstream primer (20pmol / L) 1ul, bacterial DNA template 2ul, dd H20 44ul, add to PCR amplification tube (PCR buffer, pfu enzyme and d NTPs have been added) . Reaction conditions: Denaturation at 94°C for 45s, annealing at 60°C for 60s, extension at 72°C for 150s, after 39 cycles, extension at 72°C for 10min. The amplification results were observed by 1% agarose gel electrophoresis.
[0061] (2) Cloning of the target gene and screening of positive recombinants
[0062] After electroph...
Embodiment 3
[0071] Example 3 Development of Clostridium difficile A toxin monoclonal antibody
[0072] (1) Immunized mice
[0073] A toxin genetically engineered antigen was taken out from the -20°C low-temperature refrigerator and dissolved, and then subcutaneously injected into the back of BALB / C mice (0.2ml / mouse) with an interval of 10 days. Three days before the fusion, mice were challenged with 0.15 ml of antigen in the peritoneal cavity. The immune effect was detected by ELISA method.
[0074] (2) Myeloma cells
[0075] SP2 / 0 myeloma cells: Resuscitate the SP2 / 0 cells stored in the liquid nitrogen tank, and culture them in DMEM medium containing 10% calf serum for 48-72 hours. Uniform, neatly arranged, logarithmically split, ready for fusion.
[0076] (3) cell fusion
[0077] Prepared SP2 / 0 cells and splenic lymphocytes of immunized BALB / C mice were prepared in 2×10 7 / ml and 1×10 8 / ml. Take 1ml of each and mix at room temperature. 0.8 ml of 50% PEG (molecular weight 1500...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com