Novel inhibitor of epidermal growth factor receptor (EGFR) and application thereof
An epidermal growth factor and inhibitor technology, which can be used in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., to solve problems such as hormone resistance, rapid metastasis, and resistance to radiotherapy and chemotherapy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
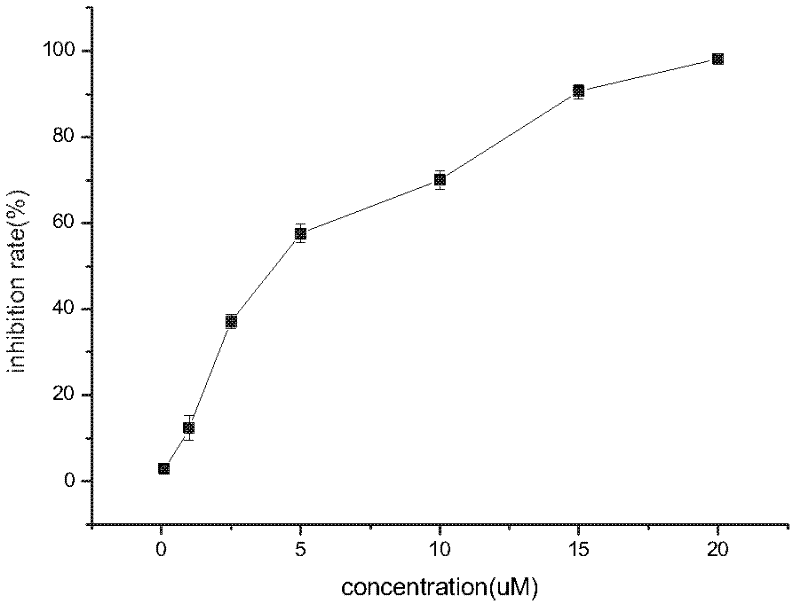
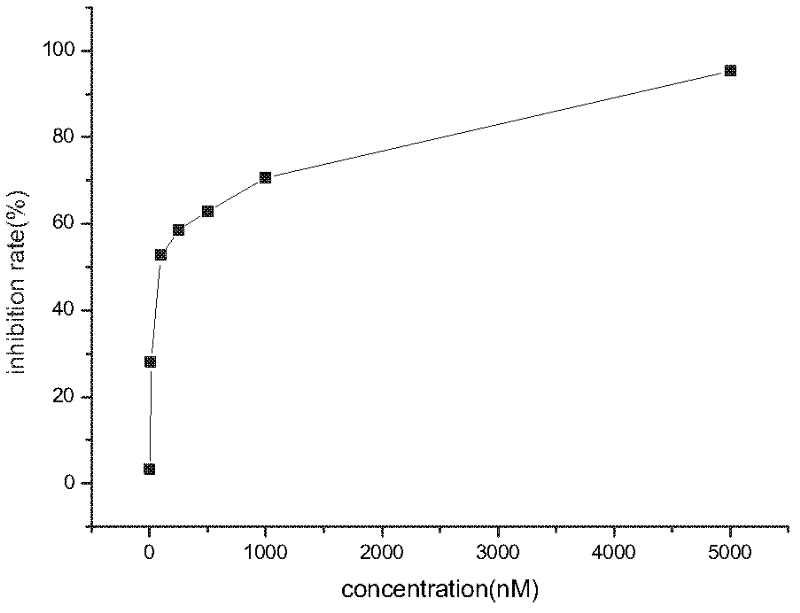
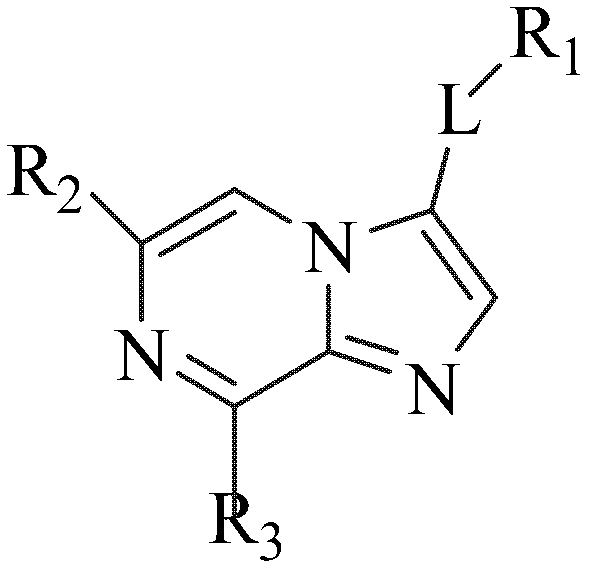
Image
Examples
Embodiment 1
[0028] 3-(1,1,3,3-Tetramethylbutylamino)-6-chloroimidazo[1,2-a]pyrazine
[0029] Under nitrogen protection, add 100 mL of a mixture of ethanol and dichloromethane and 2-amino-5-chloropyrazine (2.00 g, 15 mmol) into a 150 mL three-necked flask, stir and dissolve, then add formaldehyde (5.33, 75 mmol) and trifluoromethane Scandium sulfonate (0.74 g, 1.5 mmol). Stir at room temperature for 1 h. Add 1,1,3,3-tetramethylbutylisonitrile (2.33 g, 15 mmol) and stir at room temperature for 48 h. TLC showed that the raw material was completely reacted, the solvent was distilled off under reduced pressure, the residue was separated with water (100 mL) and ethyl acetate (150 mL), the organic layer was dried over anhydrous sodium sulfate, and concentrated to obtain the product (2.10 g, crude product). It was directly used in the next reaction without purification.
[0030] MS (EI) m / z (%): 280.2 (100%), 282.2 (33%) 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.71(s, 1H), δ: 8.46(s, 1H), δ: 7.52(s, ...
Embodiment 2
[0032] 3-Amino-6-chloroimidazo[1,2-a]pyrazine
[0033] In a 250mL round bottom flask, add 3-(1,1,3,3-tetramethylbutylamino)-6-chloroimidazo[1,2-a]pyrazine (1.00g, 3.6mmol) and dry After dissolving dichloromethane (45 mL), trifluoroacetic acid (100 mL) was added under ice-cooling, and stirred at room temperature for 1 h. After the reaction, the solvent was distilled off under reduced pressure, and saturated NaHCO 3 (200mL) after solution treatment, dichloromethane (200mL) extraction, after the organic layer is dried over anhydrous sodium sulfate, dichloromethane solvent is removed, and crude product is purified by column chromatography to obtain white solid 3-amino-6-chloroimidazo[1 , 2-a]pyrazine (0.30 g, 50%).
[0034] MS (EI) m / z (%): 168.0 (100%), 170.0 (33%) 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.62(s, 1H), δ: 8.35(s, 1H), δ: 7.20(s, 1H), δ: 6.84(s, 2H)
Embodiment 3
[0036]3-(3-Chloro-4-fluorobenzylamino)-6-chloroimidazo[1,2-a]pyrazine
[0037] Add 3-amino-6-chloroimidazo[1,2-a]pyrazine (0.20g, 1.2mmol) in a 25mL round bottom flask, (3-chloro-4-fluoroben bromide (0.40g, 1.8mmol) and 10mL of dioxane, heated and stirred under reflux for 3h. After the reaction, the solvent was distilled off, and 100mL of ethyl acetate was added. After dissolving, the solution was washed with saturated NaHCO 3 (100mL) solution was washed, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain white solid 3-(3-chloro-4-fluorobenzylamino)-6-chloroimidazo[1,2- a] Pyrazine (0.28 g, 75%).
[0038] MS (EI) m / z (%): 310.0 (100%), 312.0 (65%) 1 H NMR (DMSO-d 6 , 400MHz) δ: 8.72(s, 1H), δ: 8.45(s, 1H), δ: 7.10-6.79(m, 4H), δ: 6.68(t, 1H), δ: 4.29(d, 2H)
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap