Co-expressed molecular adjuvant enhanced divalent foot and mouth disease protein engineering vaccine
A foot-and-mouth disease, enhanced technology, applied in the field of biotechnology genetic engineering, can solve problems such as inconsistency, lack of 6th or 7th residues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Molecular adjuvants enhance the design idea of polypeptide-encoded protein in Asian type 1 and type A foot-and-mouth disease bivalent protein engineering vaccine
[0069] Integrating the genome sequences and antigenic structures of multiple Asian type 1 and type A foot-and-mouth disease virus epidemic strains AF / 72, LC / 96, AKT / 03, Asia-1-JSL, KZ / 03, HeNzk / 06 and other strains in China and Southeast Asia , epidemiological research progress, optimized the design of recombinant foot-and-mouth disease bivalent protein engineering vaccine. The present invention uses bioinformatics software to analyze the hydrophilicity, antigenicity, plasticity, surface accessibility and Garnier-Robson secondary structure of its main outer membrane proteins VP1 and VP2, and predict possible B cell antigen epitopes And on the basis of the killer T cell epitope, according to the similarity of the epitope position and amino acid sequence, analyze the common and specific antigenic ep...
Embodiment 2
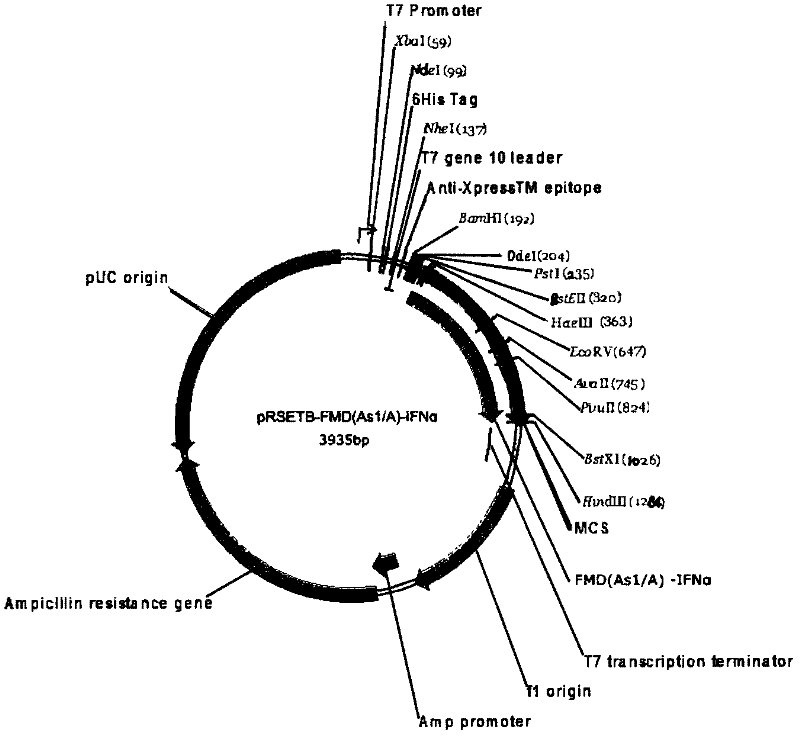
[0071] Embodiment two Escherichia coli expression vector and the construction of expression bacterial strain
[0072] The nucleotide encoding the polypeptide designed in Example 1 was sent to Shanghai Handsome Biotechnology Co., Ltd. for synthesis, and BamH I (5' end) and HindIII (3' end) restriction enzyme sites were designed at both ends of the nucleotide fragment At the same time, the two segments of multi-epitope tandem nucleotide fragments without molecular adjuvant were also designed with EcoRI (5' end) and HindIII (3' end) restriction enzyme sites, and sent to Shanghai Handsome Biotechnology Co., Ltd. synthesized as a control. After the two fragments were synthesized, they were respectively cloned into the pMD18T vector, and sequence determination confirmed that the inserted gene fragment was consistent with the designed sequence (see the sequence list). The recombinant plasmids were named pMD18T-The / A-Asia1B / Tc and pMD18T-IFNα-The / A-Asia1B / Tc, respectively. The two p...
Embodiment 3
[0076] Example 3 Fermentation, purification and emulsification of engineering bacteria
[0077] Fermentation Take the production strains, inoculate them in 2ml LB liquid medium (containing 100μg / ml ampicillin), and culture at 37°C with shaking at 180rpm for 12 hours to activate the strains. Then inoculate the shake flask with an inoculation amount of 1:100, shake and culture at 37°C until OD600=3, and then inoculate it into a fermenter at a ratio of 10%. The medium used for fermentation is a semi-synthetic medium prepared with distilled water and does not contain any antibiotics. Calibrate the dissolved oxygen and pH electrodes, start the tank to stir, the rotation speed is 300rpm, and sterilize the tank on-line. When the temperature of the culture solution in the tank drops to 37.0°C, calibrate the pH and dissolved oxygen (OD) zero point. The fermentation temperature is 37.0±0.1°C, the dissolved oxygen is controlled at about 20%, and the pH is controlled at 7.0. When the OD6...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap