N-substituted benzenepropanamide or benzenepropenamide for use in the treatment of pain and inflammation
A technology of -OR6, a compound, applied in the field of N-substituted phenylalanamide or phenylacrylamide for the treatment of pain and inflammation, capable of solving problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
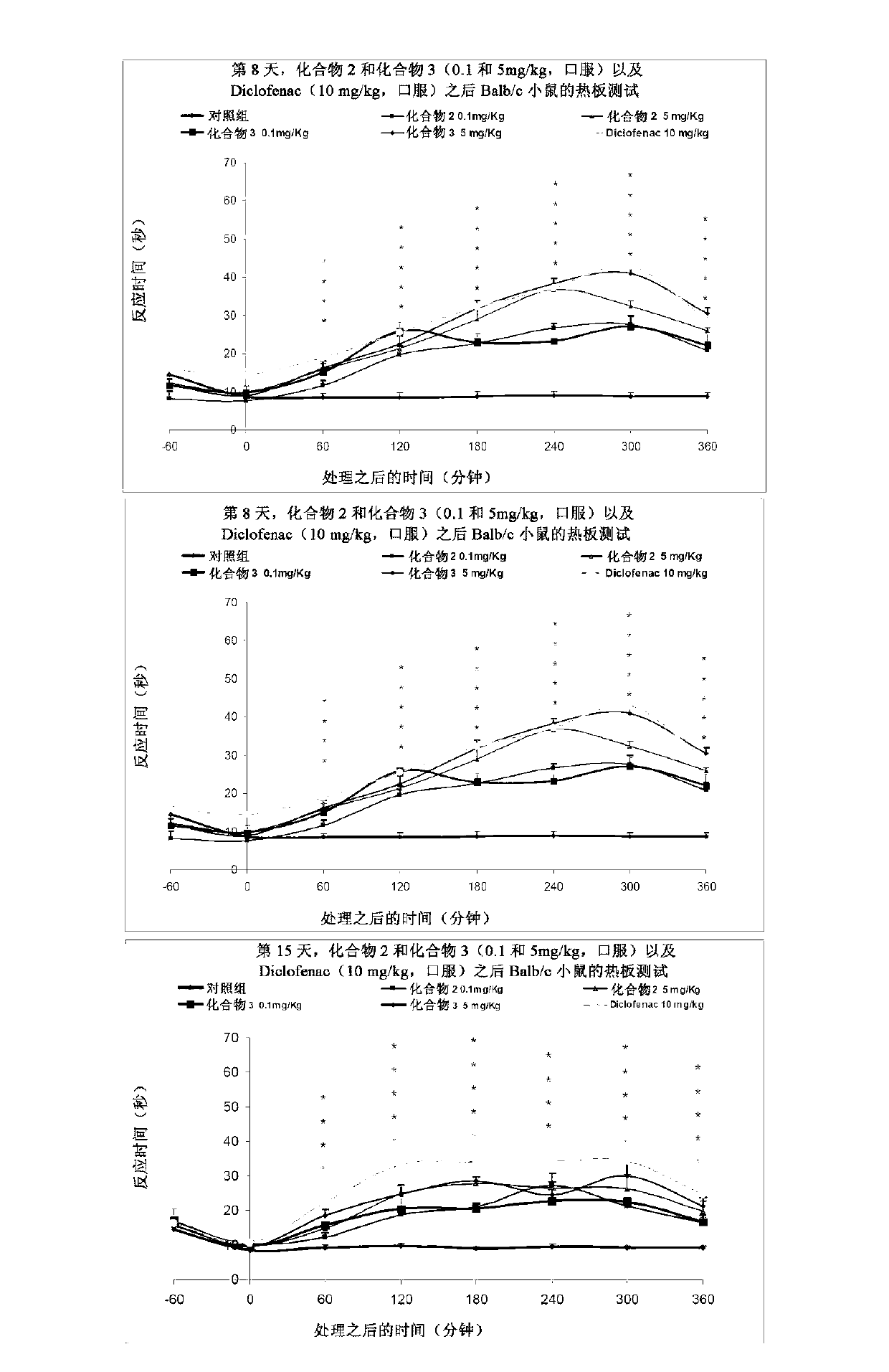
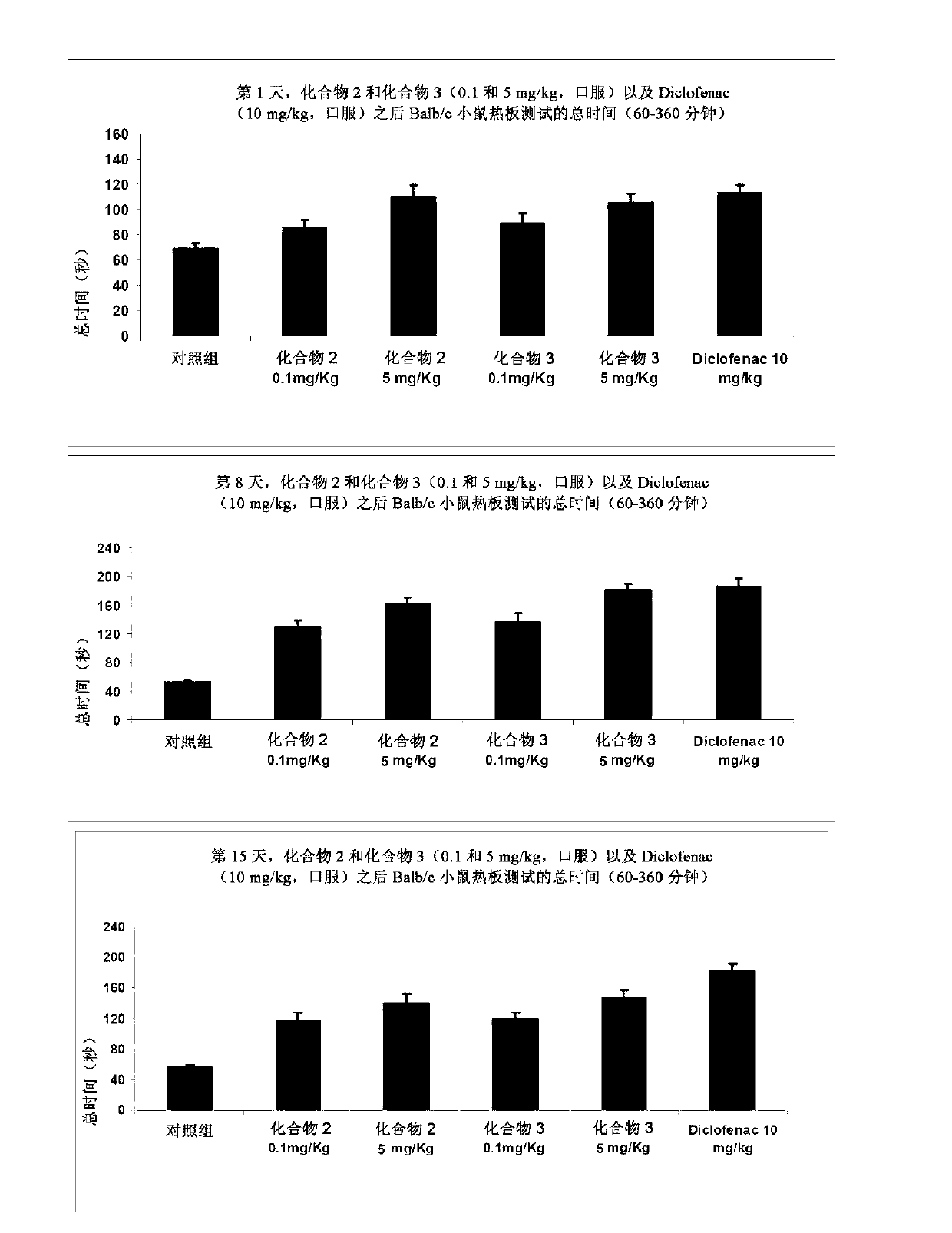
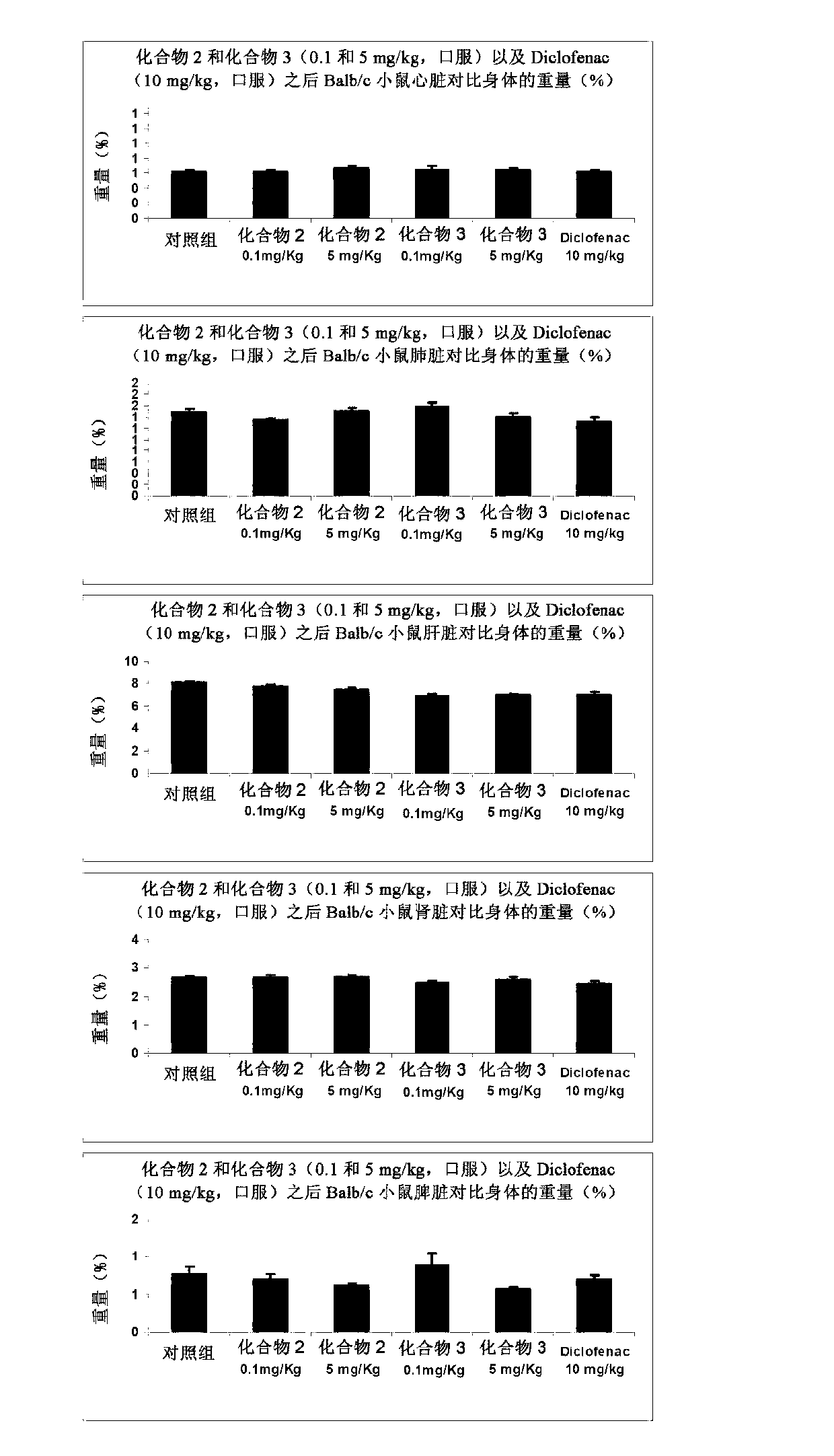
[0191] Antinociceptive effects of compound 2 and compound 3
[0192] The purpose of this study was to evaluate the antinociceptive activity of test items in the mouse hot plate test when administered subchronically. The lapse of response time to paw licking or jumping was measured following placement on a heated surface (hot plate) to determine a potential antinociceptive effect in mice.
[0193] A total of 42 Balb / c mice (12 weeks old) were used. At study initiation, mice were approximately 25 g, male. The minimum and maximum weights in the group were within ±10% of the group mean weight.
[0194] and (Perigo) compared to test compounds 2 and 3. Use DMSO solution. Six groups of mice (n=7 or n=8 mice each) were tested, the last group receiving
[0195]
[0196] Formulations prepared according to the table below were used for administration to groups of mice.
[0197]
[0198]
[0199] All groups received the drug orally daily for 16 days. Hot plate experime...
Embodiment 2
[0208] Nociceptin activity using compound 2
[0209] Using the procedure outlined in Example 1, 40 male mice (Balb / c, 9 weeks old, untested) were divided into 5 groups (8 mice each) and treated daily with the formulations shown in the table below ( 0 minutes, orally).
[0210]
[0211] Animals were assayed on a hot plate at: -60, 0, 120, 240, 360, 420 and 480 minutes. The average temperature of the hot plate is 52±1 degrees.
[0212] Figure 2a and 2b Data for these tests are presented.
Embodiment 3
[0214] Nociceptin activity using compound 2
[0215] Using the procedure outlined in Example 1, 40 male mice (Balb / c, 9 weeks old, untested) were divided into 5 groups (8 mice each) and treated daily with the formulations shown in the table below ( 0 minutes, orally).
[0216]
[0217] Animals were assayed on a hot plate at: -60, 0, 60, 120, 180, 240, 300 and 360 minutes. The average temperature of the hot plate is 52±1 degrees.
[0218] Figure 3a , 3b and 3c present the data for this test.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com