Novel amoxicillin and sulbactum composition
A technology of amoxicillin-sulbactam and amoxicillin, which is applied in the field of novel amoxicillin-sulbactam compositions, can solve the problems of very demanding preparation process conditions, increase the economic burden of patients, and protect active ingredients, and achieves the The effect of reducing production and treatment costs, reducing the frequency of medication, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
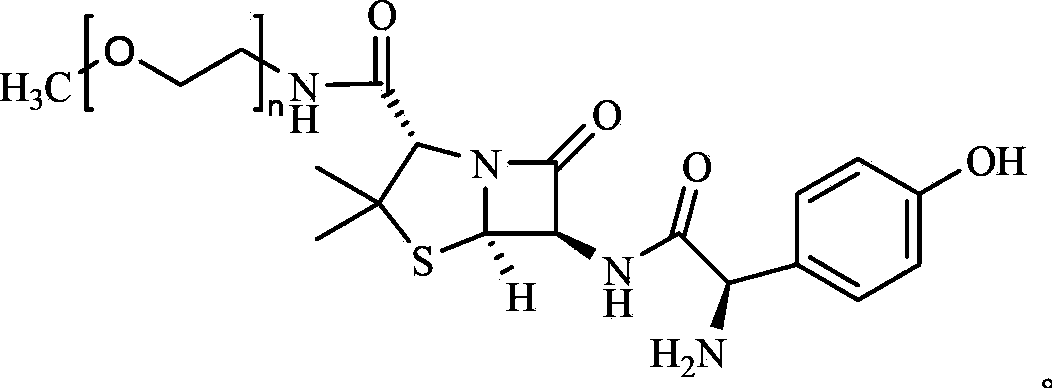
[0036] Embodiment 1 Amoxicillin-monomethoxy polyethylene glycol sulbactam compound medicine
[0037] One, the preparation of amoxicillin-monomethoxypolyethylene glycol
[0038] Amoxicillin—the reaction scheme of monomethoxypolyethylene glycol:
[0039]
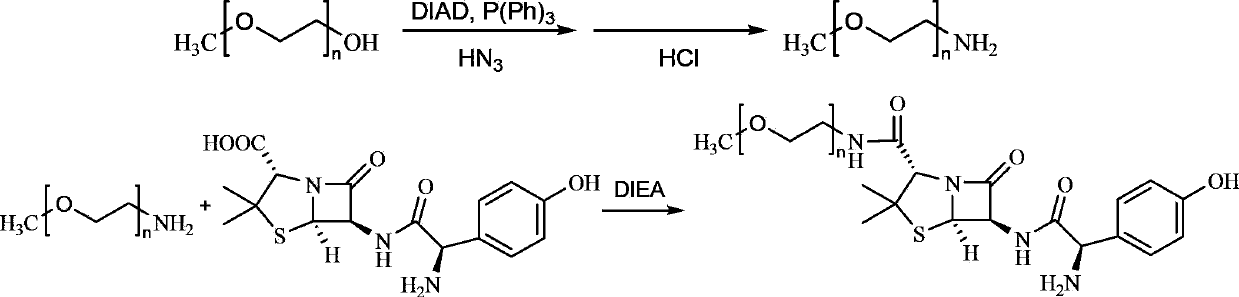
[0040] (1) Preparation of aminated monomethoxy polyethylene glycol (mPEG-NH 2 )
[0041] Add 1.5L of 1M dichloromethane solution of azide acid into 10L of 896g (0.5mol) monomethoxy polyethylene glycol (mPEG) tetrahydrofuran solution, and then add 0.3L (1.5mol) diisopropyl azodicarboxylate 2 L of tetrahydrofuran solution of ester (DIAD), and 5 L of tetrahydrofuran solution of 787 g (3.0 mol) triphenylphosphine was added to the obtained mixture, stirred at room temperature for 30 minutes, and heated at 65° C. for 2 hours. Finally, 1 L of 1M hydrochloric acid was added, heated at 65°C for another 2 hours, cooled to room temperature, filtered, and dried in vacuo. The yield is 99%.
[0042] (2) Preparation of amoxicillin-mo...
Embodiment 2
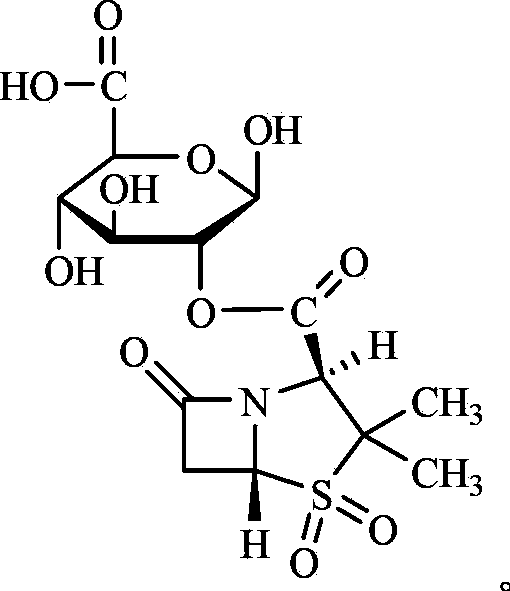
[0051] Embodiment 2 amoxicillin sodium sulbactam-glucuronate compound medicine
[0052] The compound drug involved in this embodiment is composed of unmodified amoxicillin and modified sulbactam, wherein amoxicillin sodium is used as the active ingredient. The method is as follows:
[0053] 1. Preparation of sulbactam-glucuronate
[0054] We chose to modify the carboxyl group of sulbactam to undergo esterification reaction with the hydroxyl group in glucuronic acid. Since the steric hindrance of the isomerized carboxyl group of sulbactam is relatively large, the esterification reaction only occurs on the hydroxyl group of the para-position carbon of the carboxyl group in glucuronic acid, and the product is single, and the modified product can be released quickly after entering the body. active ingredient. In addition, in order to improve the esterification activity of sulbactam, its carboxyl group is first converted into acid chloride, and then esterified with the hydroxyl ...
Embodiment 3
[0066] Embodiment 3 Amoxicillin—monomethoxypolyethylene glycol and sulbactam—preparation of glucuronate
[0067] Among the pharmaceutical ingredients involved in this example, the active ingredients are amoxicillin and sulbactam in modified forms.
[0068] One, the preparation of amoxicillin-monomethoxypolyethylene glycol
[0069] (1) Preparation of aminated monomethoxy polyethylene glycol (mPEG-NH 2 )
[0070] Add 1.5L of 1M dichloromethane solution of azide acid into 10L of 896g (0.5mol) monomethoxy polyethylene glycol (mPEG) tetrahydrofuran solution, and then add 0.3L (1.5mol) diisopropyl azodicarboxylate 2 L of tetrahydrofuran solution of ester (DIAD), and 5 L of tetrahydrofuran solution of 787 g (3.0 mol) triphenylphosphine was added to the obtained mixture, stirred at room temperature for 30 minutes, and heated at 65° C. for 2 hours. Finally, 1 L of 1M hydrochloric acid was added, heated at 65°C for another 2 hours, cooled to room temperature, filtered, and dried in v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com