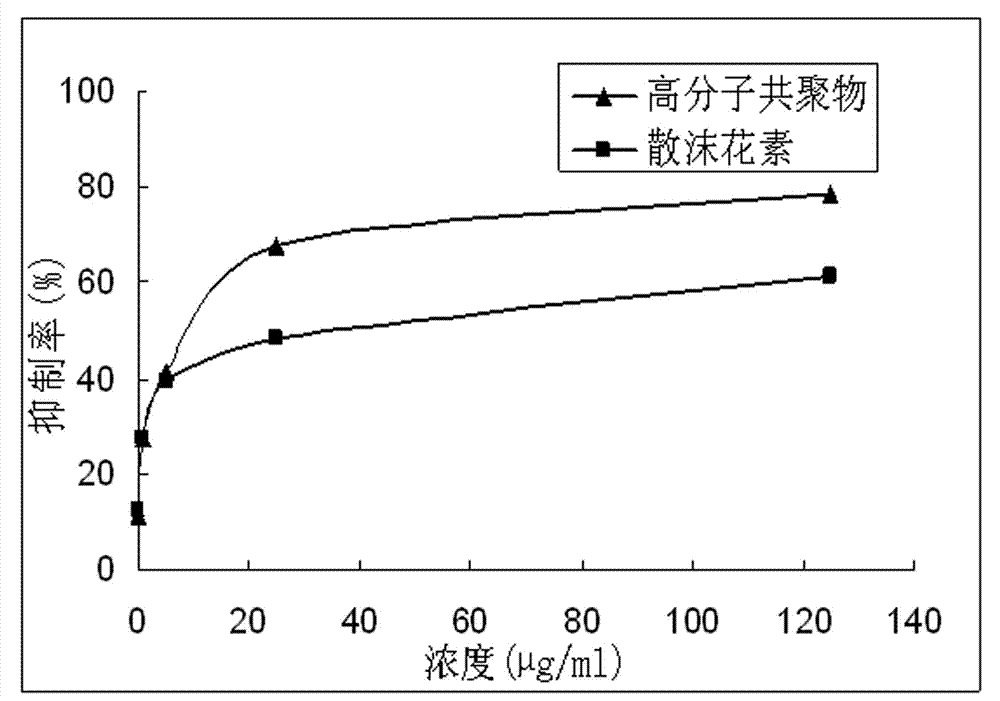
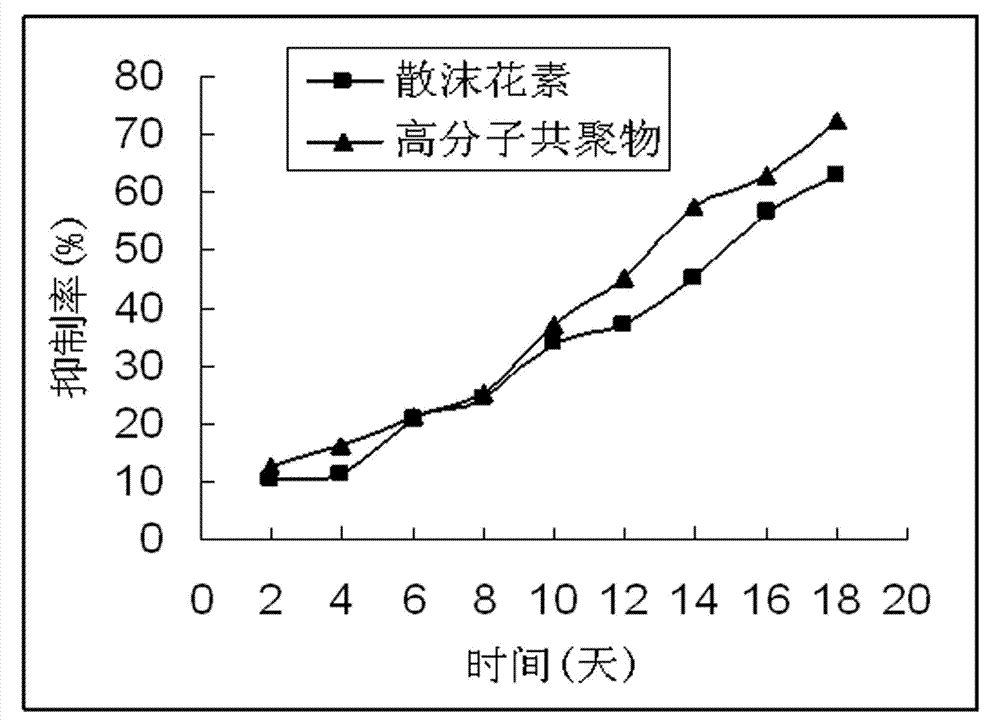
Lawsone copolymer with antineoplastic activity and preparation method thereof
A technology of anti-tumor activity and hennain, which is applied in the field of polymer chemistry, can solve the problems of toxic side effects and poor water solubility of hennain, and achieve the effects of reducing toxicity, good biological water solubility, and promoting inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Preparation of intermediate compound (a): Weigh 0.5279 g (3 mmol) of 2-hydroxy-1,4-naphthoquinone in a flask, dissolve it in 30 ml of acetone, add 0.4146 g (3 mmol) of Water K 2 CO 3 As an acid-binding agent, under the protection of nitrogen, in an ice-water bath, under constant stirring, slowly drop 0.3763 g (3.6 mmol) of methacryloyl chloride, and react at room temperature. During the reaction, TCL was used to monitor the progress of the reaction. Suction filtration after the reaction was completed, the filtrate was concentrated and the crude product was separated with a silica gel column (ethyl acetate / petroleum ether = 1 / 40 (v / v)), the product components were collected, and the solvent was evaporated to obtain 0.7432 g of a yellow solid. The rate is 88%.
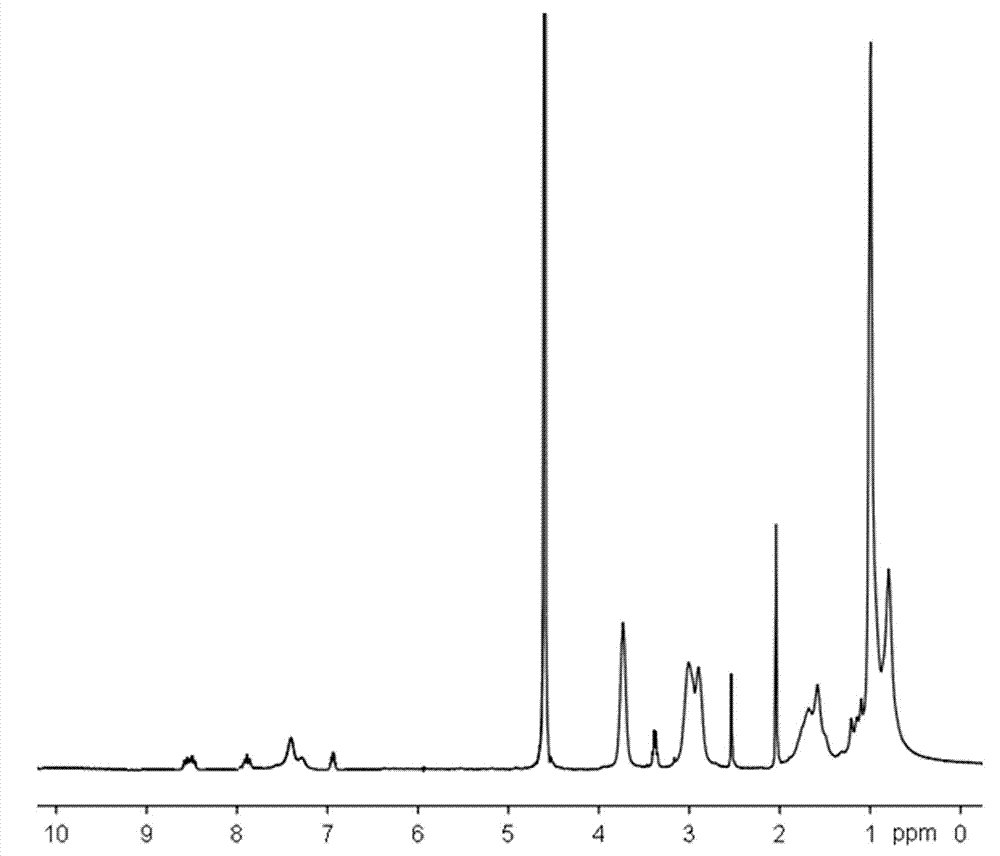
[0046] 1 H NMR (400 MHz, CCl 3 , δ, ppm): 2.111 (s, 3H, -CH 3 ), 5.915 (s, 1H, -C=CH 2 ), 6.455 (s, 1H, -C=CH 2 ), 6.862 (s, 1H, naphthyl ring), 7.809 (d, 2H, naphthyl ring), 8.138 (t, 2H, naphthyl rin...
Embodiment 2
[0050] (1) Preparation of intermediate compound: Same as Example 1.
[0051] (2) Preparation of copolymer: Weigh 0.1589 g (92%, 1.15 mmol) of HPMA into a Shleck bottle, heat to dissolve with 0.5 ml of DMSO, then add 0.5 ml of acetone; weigh 0.0242 g (8%, 0.1 mol) intermediate compound into the Shleck bottle, stir until dissolved, add 0.0145 g (8%, wt) azoisobutyronitrile (AIBN) when cooled to room temperature, vacuumize and nitrogen cycle 3~5 times, seal Finally, keep the temperature at about 55°C and react for 24 hours. Precipitate with a mixture of acetone and ether (volume ratio 7:3), filter and dissolve the precipitate with 1ml of anhydrous methanol, centrifuge with an ultrafiltration concentration centrifuge tube with a molecular weight of 3000, remove small molecules, and obtain 56mg of polymer. The yield is about 38.2%.
[0052] m n =2.4×10 4 ,M w / M n =1.16. 1 H NMR (400 MHz, D 2 O, δ, ppm): 0.785(-CH 3 ), 0.942 (-CH 3 ), 1.521-1.639 (-CH 2 -), 2.854-2.998 (...
Embodiment 3
[0054] (1) Preparation of intermediate compound: Same as Example 1.
[0055] (2) Preparation of copolymer: Weigh 0.2488 g (90%, 1.8 mmol) of HPMA into a Shleck bottle, heat and dissolve with 0.2 ml of DMSO, then add 0.2 ml of acetone; weigh 0.0484 g (10%, 0.2 mol) intermediate compound into the Shleck bottle, stir until dissolved, add 0.0238 g (8%, wt) azoisobutyronitrile (AIBN) when cooled to room temperature, vacuumize and nitrogen cycle 3~5 times, seal Finally, keep the temperature at about 55°C and react for 24 hours. Precipitate with a mixture of acetone and ether (volume ratio 7:3), filter and dissolve the precipitate with 1ml of anhydrous methanol, centrifuge with an ultrafiltration concentration centrifuge tube with a molecular weight of 3000, remove small molecules, and obtain 85mg of polymer. The yield is about 28.6%.
[0056] m n =2.4×10 4 ,M w / M n =1.16. 1 H NMR (400 MHz, D 2 O, δ, ppm): 0.808(-CH 3 ), 1.019 (-CH 3 ), 1.579-1.724 (-CH 2 -), 2.914-3.042 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap