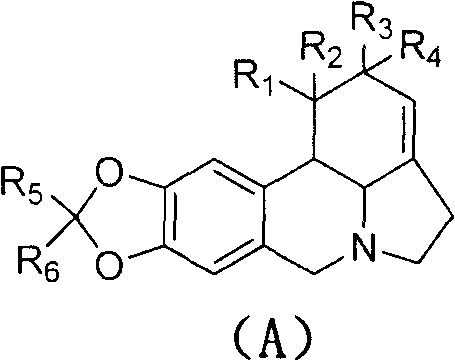
Application of lycorine compounds in the preparation of antitumor drugs
A compound, the technology of lycorine, applied in the field of lycorine compounds, can solve the problems such as the unknown mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
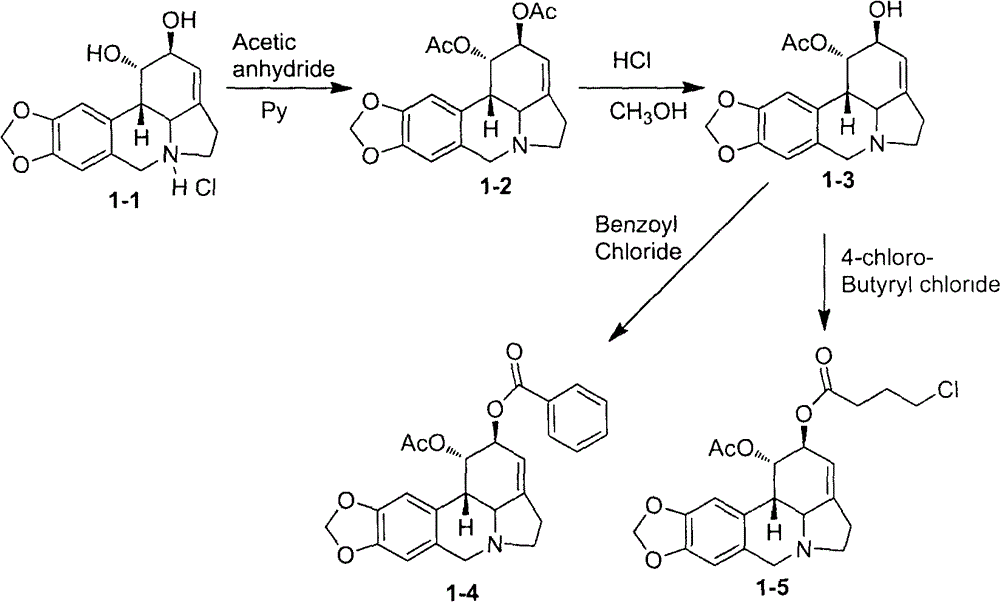
[0105] Example 1. Synthesis of 1-acetyl-2-phenyllycorine (1-4) and 1-acetyl-2-(4-chlorobutyryl) lycorine (1-5)
[0106]
[0107] Compounds 1-acetyl-2-phenyllycorine (1-4) and 1-acetyl-2-(4-chlorobutyryl)lycorine (1-5) were synthesized according to steps I-3 and I-4 .
[0108] 1-Acetyl-2-phenyllycorine (1-4): ESI-MS: m / z 434[M+H] + ; 1 HNMR (400MHz, CDCl 3 ): δ8.04(d, J=7.7Hz, 2H), 7.55(t, J=7.3Hz, 1H), 7.41(t, J=7.5Hz, 2H), 6.78(s, 1H), 6.60(s , 1H), 5.90(s, 2H), 5.67(s, 1H), 5.56(s, 1H), 4.21(d, J=14.1Hz, 1H), 3.65(d, J=14.1Hz, 1H), 3.54 -3.37(m, 1H), 3.11(d, J=10.5Hz, 1H), 2.99(d, J=10.5Hz, 1H), 2.71(s, 2H), 2.55(m, 1H), 1.99(s, 3H); 13 CNMR (100MHz, CDCl 3 ): δ170.0, 165.2, 146.7, 146.5, 145.8, 133.2, 132.1, 129.8, 129.8, 129.0, 128.4, 128.0, 126.5, 114.2, 107.4, 105.1, 101.1, 70.9, 69.0, 61.1, 56.4, 5 28.8, 20.9.
[0109] 1-Acetyl-2-(4-chlorobutyryl)lycorine (1-5): ESI-MS: m / z 434[M+H] + ; 1 HNMR (400MHz, CDCl 3 ): δ6.74(s, 1H), 6.57(s, 1H), 5.92(s, 2H), 5.7...
Embodiment 2
[0110] The synthesis of embodiment 2.2-TBS-lycorine (3-2) and 1-acetyl-2-TBS-lycorine (3-3)
[0111]
[0112] The compound 2-TBS-lycorine (3-2) was synthesized according to the steps of Scheme III-1 as a colorless oil.
[0113] 2-TBS-lycorine (3-3): ESI-MSm / z: 552 (M+H); 1 H-NMR (400MHz, CDCl 3 )δ: 6.79(s, 1H), 6.56(s, 1H), 5.89(s, 2H), 5.39(s, 1H), 4.37(s, 1H), 4.24(s, 1H), 4.10(d, J =14.0Hz, 1H), 3.45(d, J=14.0Hz, 1H), 3.30(m, 1H), 2.77(d, J=10.4Hz, 1H), 2.68(d, J=10.4Hz, 1H), 2.57 (m, 2H), 2.30 (m, 1H), 0.87 (s, 9H), 0.12 (s, 3H), 0.09 (s, 3H). 13 C-NMR (100MHz, CDCl 3 )δ: 146.3, 146.0, 141.8, 130.1, 128.2, 118.3, 107.6, 104.6, 100.8, 72.5, 72.0, 60.9, 57.1, 53.9, 40.8, 28.6, 25.9, 18.1, -4.4, -4.7.
[0114] The compound 1-methylsulfonyl-2-TBS-lycorine (3-3) was synthesized according to the steps of Scheme III-2 as a pale yellow solid.
[0115] 1-Methanesulfonyl-2-TBS-lycorine (3-3): ESI-MSm / z: 444 (M+H); 1 H-NMR (400MHz, CDCl 3 )δ: 6.73(s, 1H), 6.57(s, 1H), 5.91...
Embodiment 3
[0116] Example 3. Synthesis of 1-acetyl-2-carbonyl-lycorine (2-2), 2-carbonyl-lycorine (2-3) and 1-butyryl-2-carbonyl-lycorine.
[0117]
[0118] Compound 1-acetyl-2-carbonyl-lycorine (2-2) was synthesized according to the steps of scheme II-1 as a white solid.
[0119] 1-Acetyl-2-carbonyl-lycorine (2-2): ESI-MSm / z: 328 (M+H); 1 H-NMR (400MHz, CDCl 3 )δ: 6.67(s, 1H), 6.52(s, 1H), 5.94(s, 1H), 5.93(s, 1H), 5.89-5.85(m, 2H), 4.12(d, J=14.0Hz, 1H ), 3.56(d, J=14.0Hz, 1H), 3.41(m, 1H), 3.21(d, J=10.4Hz, 1H), 3.13(d, J=9.6Hz, 1H), 2.82(dm, 2H ), 2.48(q, J=8.6Hz, 1H), 1.91(s, 3H). 13 C-NMR (100MHz, CDCl 3 )δ: 192.9, 169.5, 169.1, 146.7, 146.6, 128.9, 125.2, 120.3, 107.3, 105.3, 101.0, 69.0, 62.3, 56.3, 53.2, 45.5, 30.0, 20.7.
[0120] The compound 2-carbonyl-lycorine (2-3) was synthesized according to the steps of Scheme II-2 as a pale yellow solid.
[0121] 2-Carbonyl-lycorine (2-3): ESI-MSm / z: 286 (M+H); 1 H-NMR (400MHz, CDCl 3 )δ: 6.75(s, 1H), 6.59(s, 1H), 5.99-5.89(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com