Use of (s) - esmolol for controlling venous irritation associated with the treatment of a cardiac disorder
一种刺激性、用途的技术,应用在心血管系统疾病、酯类有效成分、退热药等方向,能够解决降低应激患者心率等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
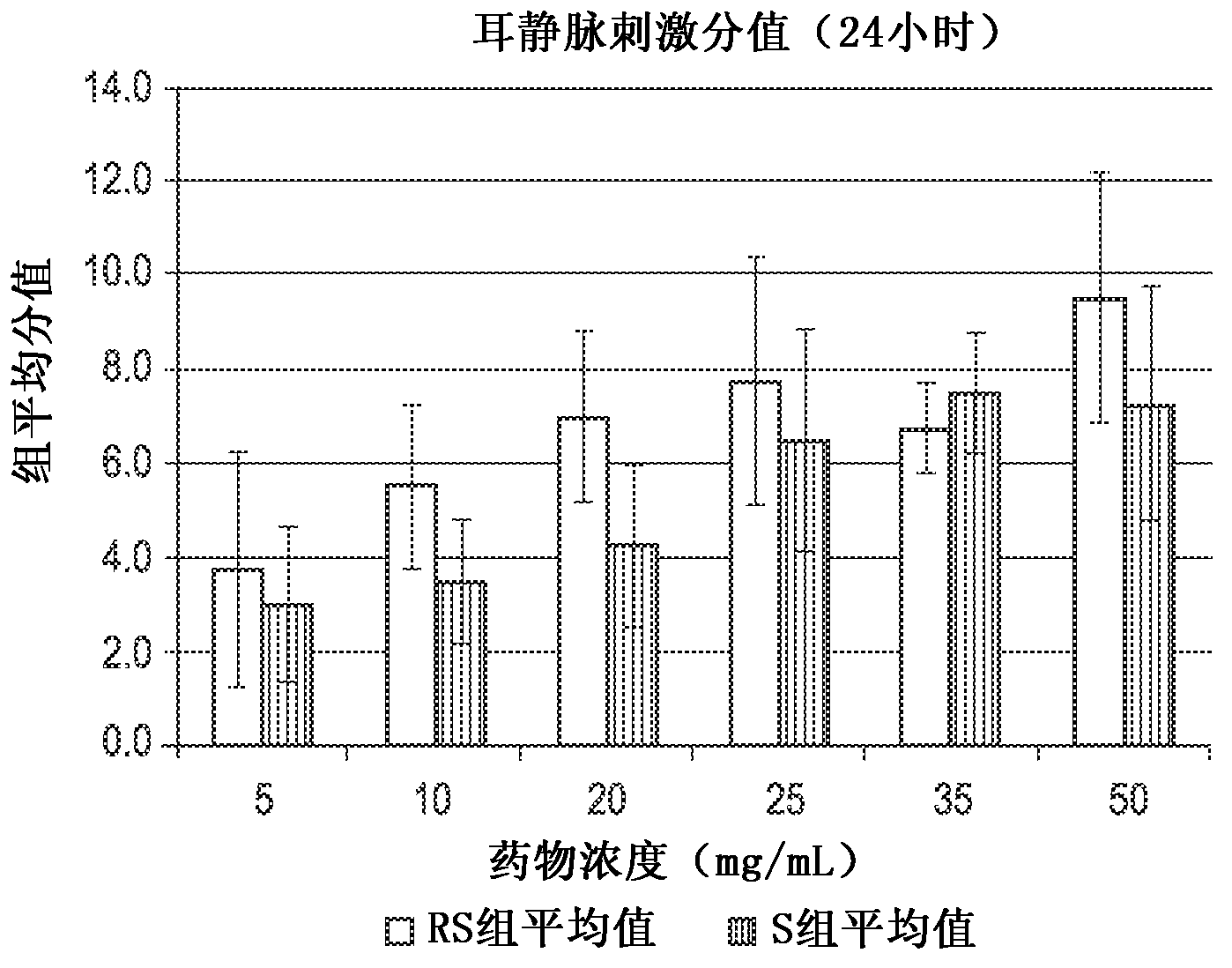
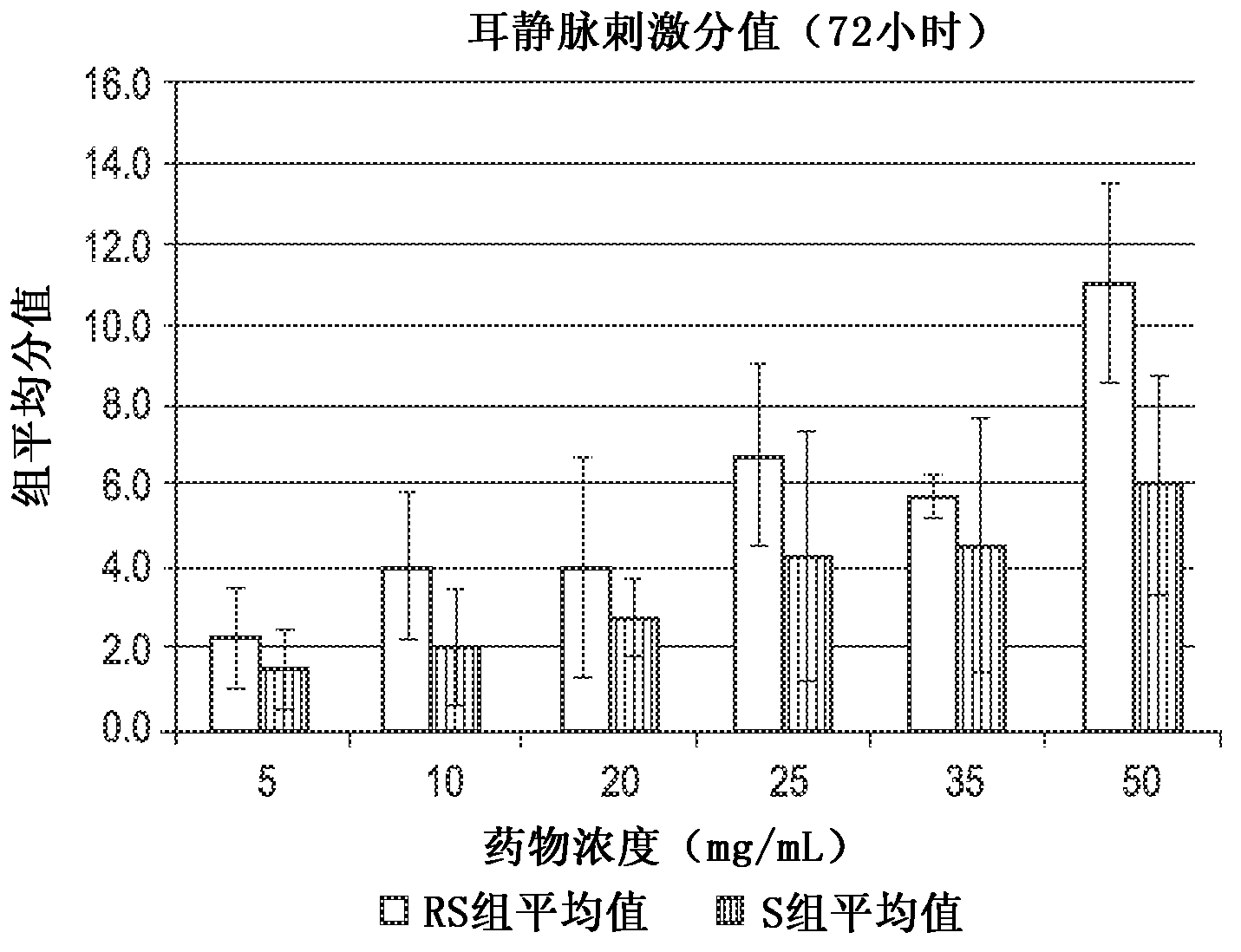
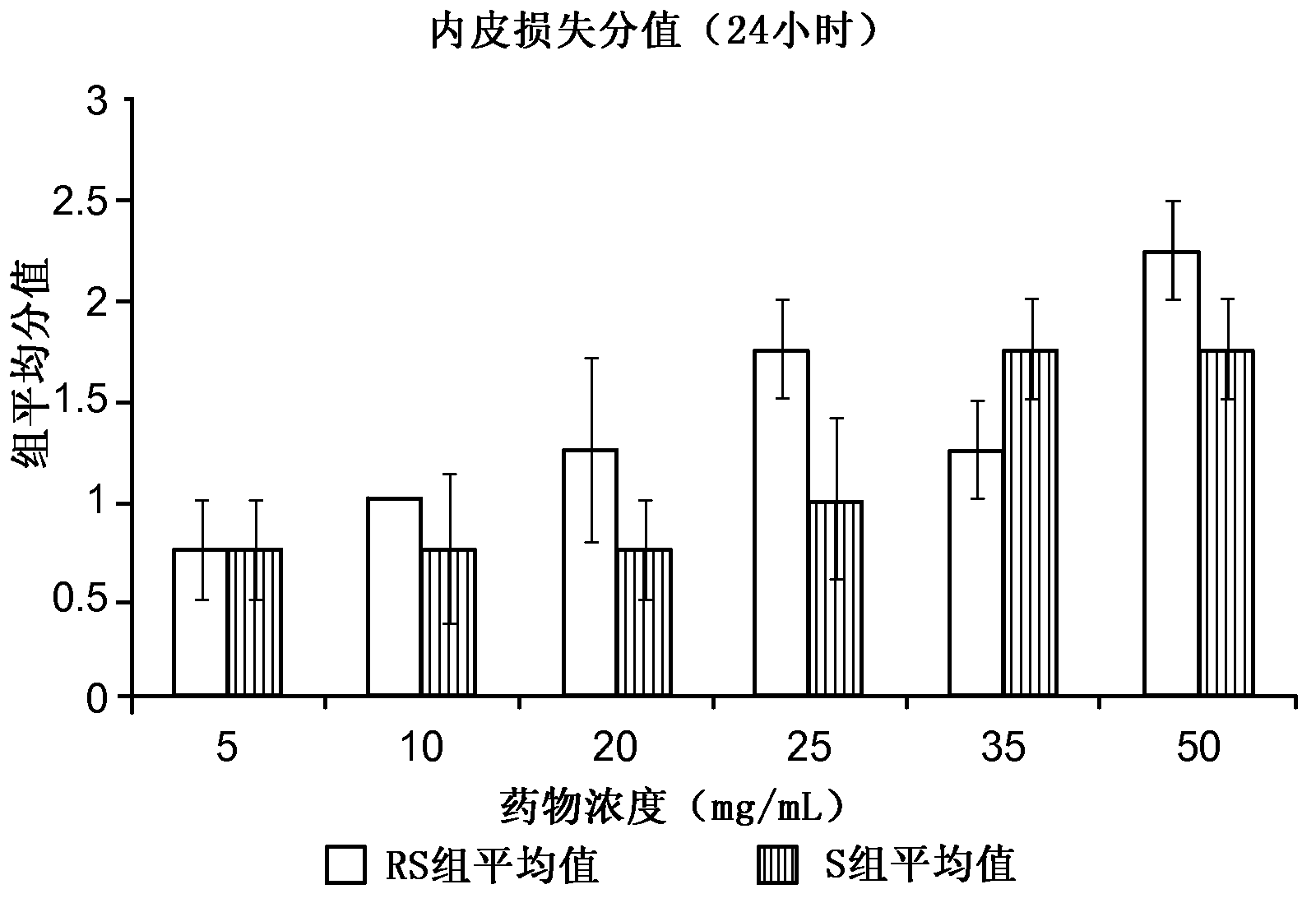
[0073] The rabbit ear vein irritation test was used to evaluate the relative potential of the following compositions to cause venous irritation in human patients: (i) containing 3-[4-(2-hydroxy-3-isopropylamino)propoxy]phenyl The composition of the racemic mixture of methyl propionate hydrochloride ("racemate" in Table 1), and (ii) containing (S)-3-[4-(2-hydroxy-3-isopropyl) (R)-3-[4-(2-hydroxy-3-isopropylamino)propoxy]phenylpropionate hydrochloride Composition of acid methyl ester hydrochloride ("S-isomer" in Table 1). The choice of rabbit as the test system is based on the established knowledge that it can be accepted for vascular stimulation research. Hessov and Bojen- , Europ J Intens Care Med, 2:97-101 (1976); Hessov et al., Intens Care Med, 5:79-81 (1979); Johnson et al., J Oral Maxil Surg, 47:819-822 (1989); Hoover Et al. Fundam Appl Toxicol, 14:589-597 (1990). The rabbit model may be more sensitive than humans, but nonetheless is still suitable for therapeutic conce...
Embodiment 2
[0105] The study described in Example 1 was extended to evaluate the relative potential of the following compositions to cause venous irritation in human patients: (i) containing 3-[4-(2-hydroxy-3-isopropylamino)propoxy] The composition of the racemic mixture of phenylpropionic acid methyl ester hydrochloride ("racemate" in Table 1), and (ii) containing 88mg / mL or 133mg / mL(S)-3-[4-( 2-hydroxy-3-isopropylamino)propoxy]phenylpropionic acid methyl ester hydrochloride and substantially free of (R)-3-[4-(2-hydroxy-3-isopropylamino) ) Composition of propoxy]phenylpropionic acid methyl ester hydrochloride ("S-isomer" in Table 1).
[0106] Sixteen 15-week-old female New Zealand white rabbits weighing in the range of 2–3 kg were tested. The rabbits are reported to be free of specific pathogens, as determined by serological, bacteriological and parasitological tests. Upon arrival, the animals were quarantined for 7 days. Only rabbits showing no signs of clinical disease were used in thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com