Anti-BLyS antibody
一种抗体、序列的技术,应用在抗体、抗体医疗成分、抗炎剂等方向,能够解决毒性强、疗效差、特异性差等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
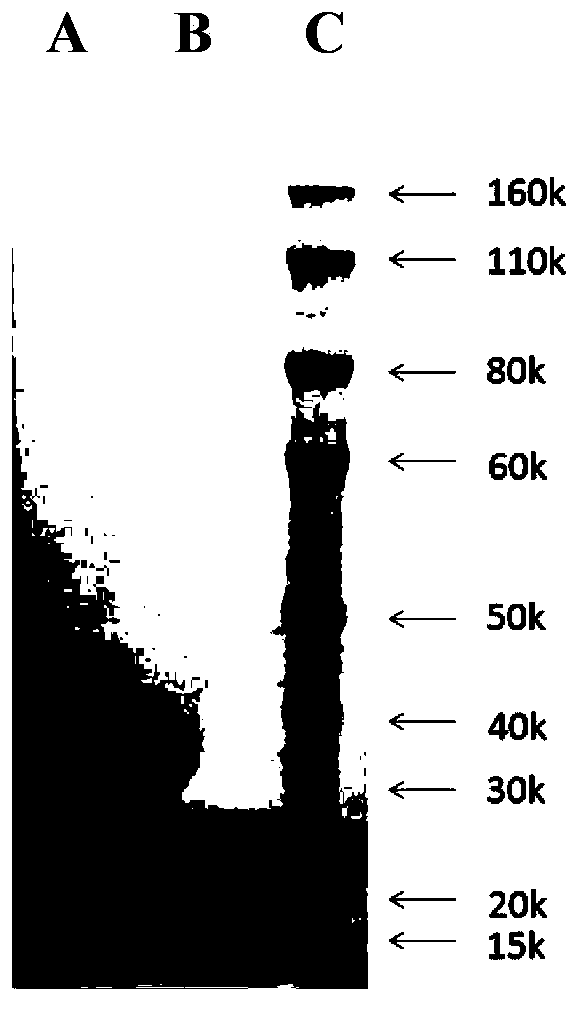
[0062] Example 1: Cloning and expression of human BLyS gene
[0063] Peripheral blood of healthy people was isolated, and total RNA was extracted and purified with a commercial RNA kit (Qiagen). Using purified total RNA as a template, reverse transcription to synthesize cDNA first-strand cDNA. The reaction system is as follows:
[0064]
[0065] Add sterilized distilled water to make up the total amount of the system 20 μL.
[0066] The reaction conditions were: 30°C for 10 minutes, 42°C for 30 minutes, 99°C for 5 minutes, and 4°C for 5 minutes. After the reaction was completed, ice bath for 5 min.
[0067] According to the full-length DNA sequence of human BLyS, the cloned secreted BLyS primers P1 and P2 were designed and synthesized, and the cDNA synthesized by reverse transcription was used as the template to carry out PCR amplification with the upstream primer P1 and the downstream primer P2. The nucleotide sequences of P1 and P2 are as follows:
[0068] P1: 5'tacga...
Embodiment 2
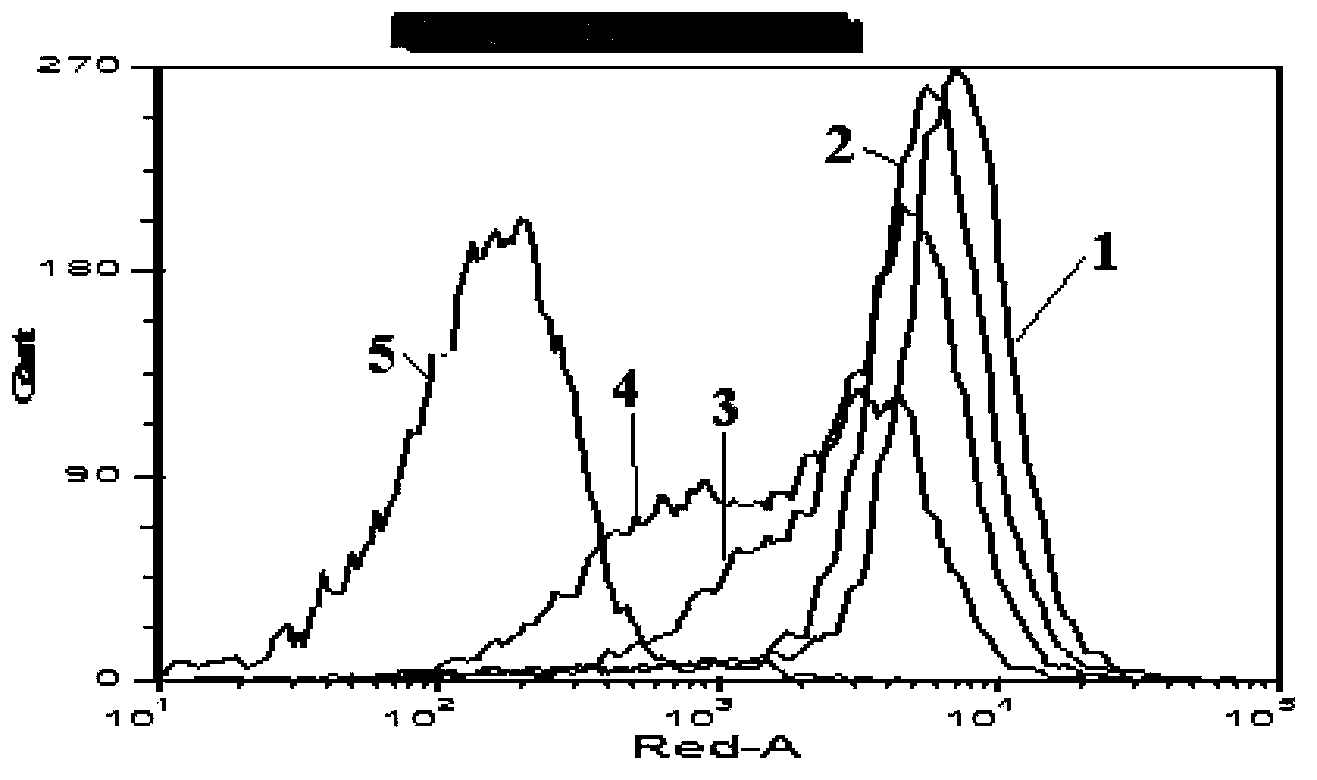
[0077] Example 2: Detection of the ability to bind to receptors on BJAB cells
[0078] 1. Biotin-labeled his-hBLyS recombinant protein
[0079] The his-hBLyS recombinant protein purified in Example 1 was mixed with biotin-xx-NHS dissolved in DMSO at a weight-to-volume ratio of 1:4 in ng / mL, placed at room temperature for 1 hour, and the reaction mixture was passed through a gel column , separation of biotin-labeled his-hBLyS and free biotin.
[0080] 2. Biotin-labeled his-hBLyS recombinant protein combined with BJAB cells
[0081] The isolated biotin-labeled his-hBLyS recombinant protein was divided into four different concentration groups, 25ng / mL, 50ng / mL, 100ng / mL and 200ng / mL, respectively with 1×10 6 The Burkitt's lymphoma cells (BJAB) cells were mixed, incubated at 4°C for 15 minutes, washed 3 times with PBS, added with straptavidin allophycocyanin (SA-APC) to a final concentration of 0.2 μg / mL, and incubated at 4°C for 20 minutes. After washing 3 times with PBS, the ...
Embodiment 3
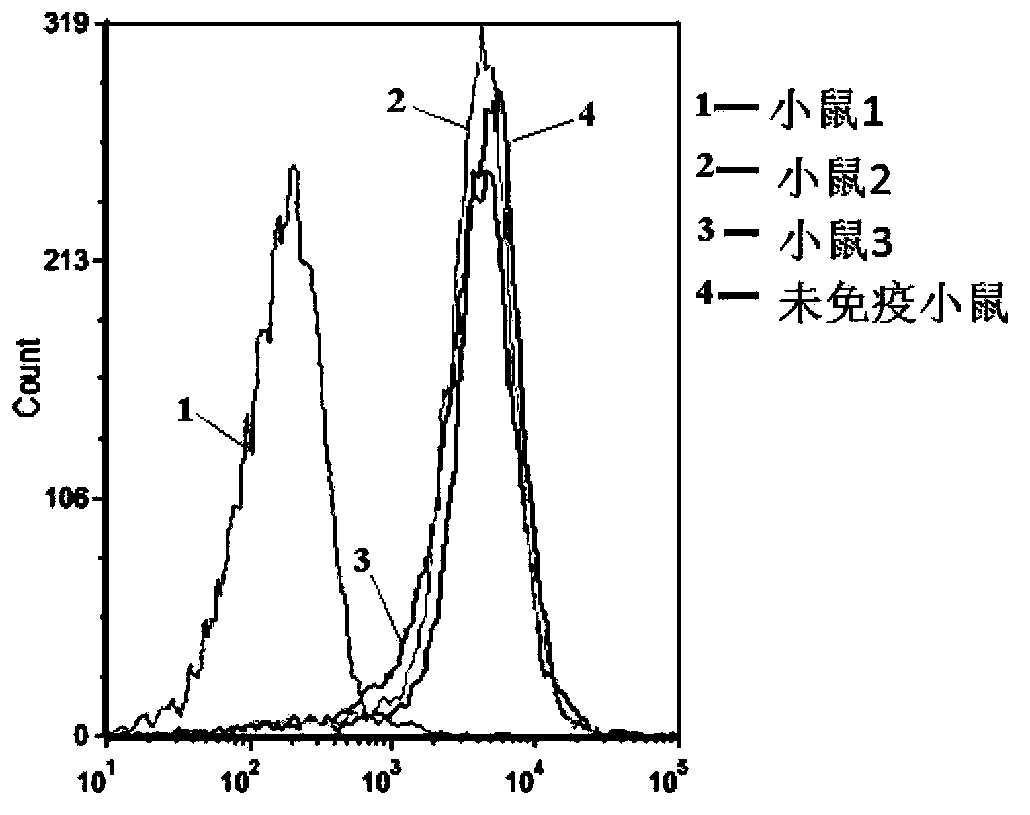
[0083] Example 3: Immunization of mice
[0084] The his-hBLyS recombinant protein purified in Example 1 was mixed with an equal amount of immune adjuvant (Freund's adjuvant) as an antigen, and 4 6-week-old female FVB mice were taken, 3 of which were immunized and the other was subjected to control simulation. experiment. After the primary immunization, booster immunizations were performed once a week. Before the last booster immunization, blood was drawn from the hearts of immunized mice, and their serum was mixed with biotin-labeled his-hBLyS recombinant protein (concentration 50ng / mL) and incubated at room temperature for 20 minutes. The mixture was then incubated with BJAB cells at 4°C for 15 minutes, and after 3 washes with physiological saline, 0.2 μg / mL of straptavidin allophycocyanin was added and incubated at 4°C for 15 minutes. After eluting with normal saline for 3 times, the samples were flow cytometer to detect whether the serum of the immunized mice could inhibi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com