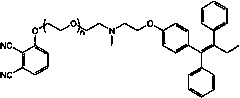
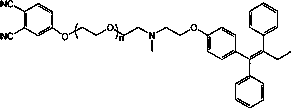
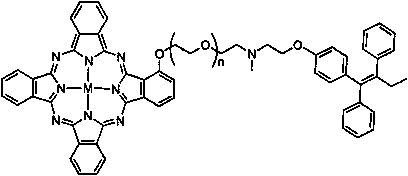
A molecularly targeted anticancer photosensitizer tamoxifen-phthalocyanine conjugate and preparation method thereof
A tamoxifen, molecular targeting technology, applied in the field of organic and metal coordination compound synthesis, to achieve the effect of definite composition, favorable for industrial production, and single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 (M=Zn (II), n=3, α-position monosubstituted)
[0041] 1) Add 0.57g (1.90mmol) of the compound in sequence to a 50mL reaction bottle equipped with a magnetic stirring device, an airway device and a reflux condensing device 2 , 0.5g (1.30mmol) compound 1 and 30 mL of acetonitrile, stirred until completely dissolved, under the protection of nitrogen, 0.48 g (3.44 mmol) of anhydrous potassium carbonate was added to the reaction flask, and the reaction was carried out at 85°C for 12 hours. After the reaction was over, the acetonitrile was removed by rotation under reduced pressure, and the 2 Cl 2 Extracted three times, dried over anhydrous magnesium sulfate, evaporated under reduced pressure to remove CH 2 Cl 2 , to CH 2 Cl 2 :CH 3 A mixed solution of OH=30:1 (V / V) was used as an eluent, and passed through a silica gel column to obtain about 0.35 g of a viscous light yellow liquid (ie compound 3), with a yield of about 51.81%. 1 HNMR (400MHz, CDCl 3 ): δ...
Embodiment 2
[0044] Example 2 (M=Zn (II), n=3, β-position monosubstituted)
[0045] 1) Add 2.34g (7.80mmol) of the compound in sequence to a 50mL reaction bottle equipped with a magnetic stirring device, an airway device and a reflux condensing device 2 , 1.0g (2.6mmol) compound 1 and 50mL of acetonitrile, stirred until completely dissolved, under nitrogen protection, 0.96g (6.88mmol) of anhydrous potassium carbonate was added to the reaction flask, and reacted at 85°C for 20 hours. After the reaction was over, the acetonitrile was removed by rotation under reduced pressure, and the 2 Cl 2 Extracted three times, dried over anhydrous magnesium sulfate, evaporated under reduced pressure to remove CH 2 Cl 2 , to CH 2 Cl 2 :CH 3 The mixed solution of OH=30:1 (V / V) was used as the eluent and passed through a silica gel column to obtain about 0.8 g of a viscous light yellow liquid (namely compound 3), with a yield of about 59.20%. 1 HNMR (400MHz, CDCl 3 ): δ 0.92(m,3H,CH 3 ),2.38(s,3...
Embodiment 3
[0048] Example 3 (M=Al, n=3, α single substitution)
[0049] 1) Add 1.0g (3.33mmol) of the compound in sequence to a 50mL reaction bottle equipped with a magnetic stirring device, an airway device and a reflux condensing device 2 , 0.5g (1.30mmol) compound 1 and 40mL of acetonitrile, stirred until completely dissolved, under the protection of nitrogen, 0.48g (3.44mmol) of anhydrous potassium carbonate was added to the reaction flask, and the reaction was carried out at 85°C for 8 hours. After the reaction was over, the acetonitrile was removed by rotation under reduced pressure, and the 2 Cl 2 Extracted three times, dried over anhydrous magnesium sulfate, evaporated under reduced pressure to remove CH 2 Cl 2 , to CH 2 Cl 2 :CH 3 A mixed solution of OH=30:1 (V / V) was used as an eluent, and passed through a silica gel column to obtain about 0.48 g of a viscous light yellow liquid (namely compound 3), with a yield of about 68.10%. 1 HNMR (400MHz, CDCl 3 ): δ 0.92(m,3H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com