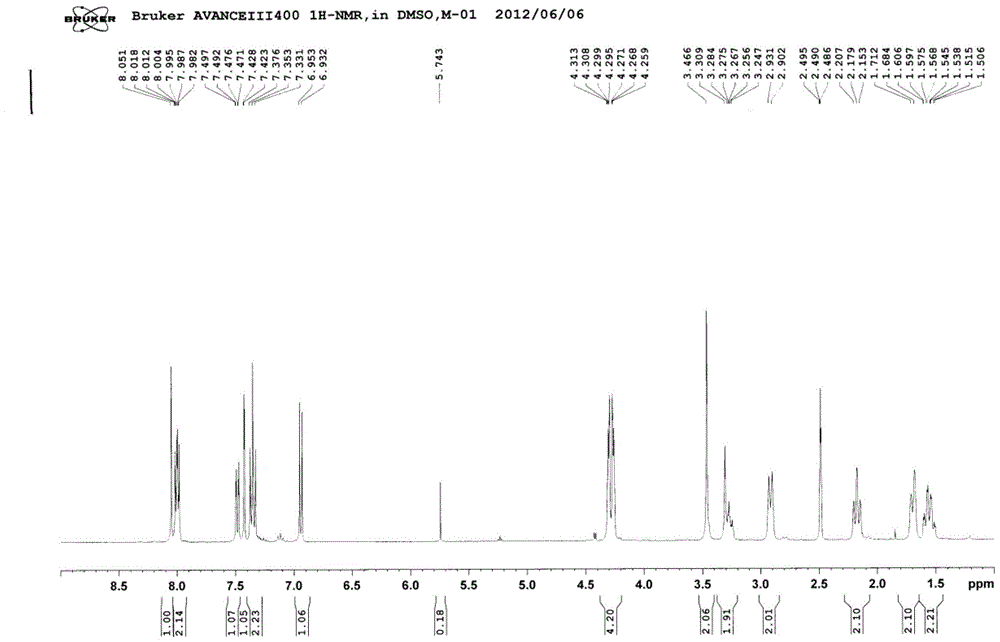
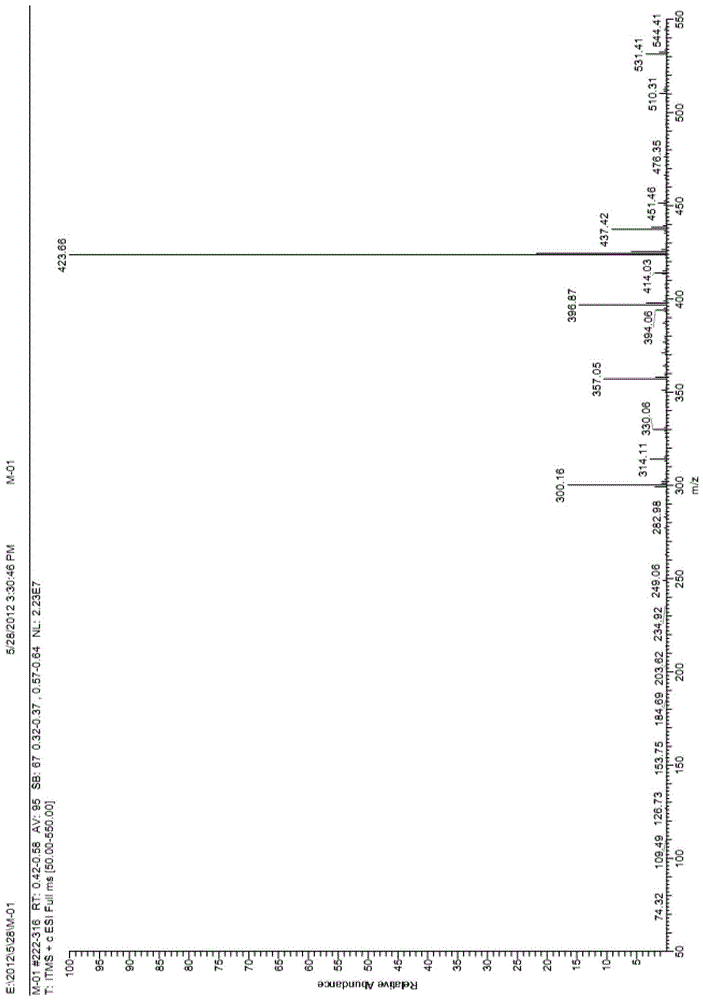
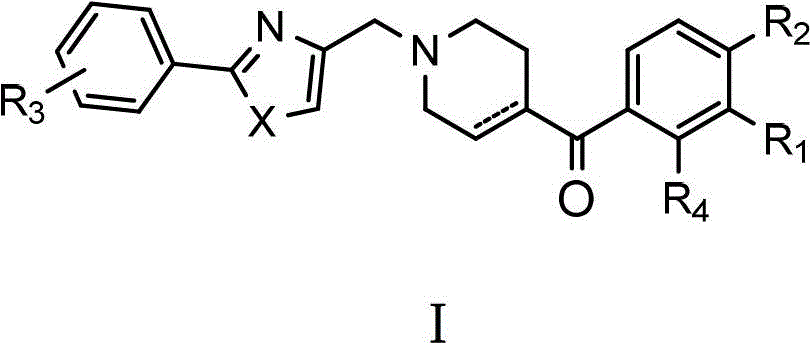
Substituted n-((1',3'-heterazol-4'-yl)-methyl)-4-benzoyl hexahydropyridine compounds and uses thereof
A technology of compounds and uses, applied in the field of preparing anti-tuberculosis drugs, which can solve the problems of no new anti-tuberculosis drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1: Synthesis of N-acetylpiperidine-4-carboxylic acid
[0079]
[0080] Take 20.0g (154.8mmol) of piperidinecarboxylic acid, 30ml (317.4mmol) of acetic anhydride, and 12.5ml (155.3mmol) of pyridine in a 100ml round bottom flask, and reflux at 140°C for 2 hours to obtain a brown solution. Most of the solvent was evaporated under reduced pressure to obtain an emulsion, which was cooled. Add 40ml of ethyl acetate-ether (v / v=1:1) solution to the above emulsion, stir to precipitate a solid, filter with suction, and wash with ethyl acetate-ether (v / v=1:1) solution to obtain a white Solid, 20.9g, m.p.174~176℃, yield 78.9%: 1 H NMR (400MHz, CD 3 OD): δ=4.293(m,1H),3.805(m,1H),3.145(m,1H),2.789(m,1H),2.525(m,1H),2.036(s,3H),1.855(m ,2H),1.516(m,2H);MS(ESI):m / z[M+H] + 172.14.
Embodiment 2
[0081] Example 2: Synthesis of N-acetylpiperidin-4-ylbenzo-1',4'-dioxan-3-ylmethanone
[0082]
[0083] Take 5ml (0.076mol) of thionyl chloride and 30ml of dichloromethane in a 10ml round-bottomed flask, and add 5.0g (0.033mol) of synthetic N-acetylpiperidine-4-carboxylic acid under ice-bath and stirring conditions. Add 1 drop of DMF dropwise, and react at 40°C for 3 hours. The solvent was evaporated under reduced pressure to obtain a brown solid, which was eluted with 25ml of 1,2-dichloroethane in batches and placed in a constant pressure dropping funnel for later use.
[0084] Take 6.67g (0.05mol) of aluminum trichloride and 4.49g (0.033mol) of benzo-1,4-dioxane into a 100ml three-necked flask, add 12.5ml of 1,2-dichloroethane, drop at 0°C Add the above-mentioned acid chloride solution, and heat the reaction at 65°C for 16 hours after the addition. Allow to cool, pour into 100 ml of ice water, separate the organic phase, wash the water phase with 25 ml of dichloromethan...
Embodiment 3
[0085] Example 3: Synthesis of piperidin-4-ylbenzo-1',4'-dioxan-3-yl ketone hydrochloride
[0086]
[0087] Take 3.13g (0.01mol) of N-formylpiperidin-4-ylbenzo-1',4'-dioxan-3-ylmethanone in a 100ml round bottom flask, dissolve it in 25ml tetrahydrofuran, and then add 3mol / L hydrochloric acid solution 40ml, reflux at 100°C for 10 hours. The solvent was evaporated under reduced pressure to obtain a brown solid, which was recrystallized from methanol-ether to obtain 2.64 g of off-white solid, m.p.211~213°C, yield 93.3%: 1 H NMR (400MHz, DMSO-d 6):7.538~7.517(dd,J=8.4Hz,J=2.0Hz,1H),7.509(d,J=2.0Hz,1H),4.333~4.271(m,4H),3.660(m,1H),3.005 (t,2H),1.891(d,2H),1.700(m,2H); MS(ESI):m / z[M-HCl+H] + 248.52.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com