Pyronaridine compounds and applications thereof
A kind of pyronaridine and compound technology, applied in the field of antimalarial pyronaridine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0059] Example 12-methoxy-7-chloro-10-(3',5'-bis 3"-methyltetrahydropyrromethene-4'-hydroxyphenyl)aminobenzo[b]-1 , Preparation of 5-naphthyridine (compound PYR_90)
[0060] Mix 0.003 mole of 2-methoxy-7-chloro-10-(4'-hydroxyphenyl)aminobenzo[b]-1,5-naphthyridine with 5 ml of formaldehyde solution and 0.008 mole of 3-formaldehyde Dipyrrole was heated under reflux in 10 ml of 95% ethanol for 6 hours, filtered while it was hot, the filtrate was left to cool, and crystals were precipitated, collected crystals, recrystallized with acetone, weighing 0.99 g, yield 60.4%, melting point 168-170 ° C, 1 H-NMR ((CD 3 ) 2 SO)δ:9.05(s,1H,Ar-NH),8.21-6.71(m,7H,Ar-H),4.04(s,3H,OCH 3 ), 3.36 (s, 4H, Ar- CH 2 N),2.86(m,2H,N( CH 2 CH 2 CHCH 2 )CH 3 ),2.50(m,2H,N(CH 2 CH 2 CH CH 2 ) CH 3 ),2.16(m,2H,N( CH 2 CH 2 CHCH 2 )CH 3 ),1.94(m,2H,N(CH 2 CH 2 CH CH 2 )CH 3 ),1.67(m,4H,N(CH 2 CH 2 CHCH 2 ) CH 3 ),1.60(m,2H,N(CH 2 CH 2 CH CH 2 )CH 3 ),1.05(d,6H,N...
Embodiment 22
[0061] Example 22-Methoxy-7-chloro-10-(3',5'-bis-hexahydropiperidinium-4'-hydroxyphenyl)aminobenzo[b]-1,5-naphthalene Preparation of pyridine (compound PYR_98)
[0062] In addition to using piperidine instead of 3-methylpyrrole, the compound 2-methoxy-7-chloro-10-(3',5'-bis-hexahydropiperidinylmethine was synthesized according to the similar synthetic route in Example 1 -4'-Hydroxyphenyl)aminobenzo[b]-1,5-naphthyridine (compound PYR98), yield 53.4%, melting point 165-166℃ 1H-NMR ((CD 3 ) 2 SO)δ:9.02(s,1H,Ar-NH),8.20-6.81(m,7H,Ar-H),4.04(s,3H,OCH 3 ), 3.38 (s, 4H, Ar- CH 2 N),2.86(m,8H,N( CH 2 CH 2 CH 2 CH 2 CH 2 )),1.92(m,8H,N(CH 2 CH 2 CH 2 CH 2 CH 2 )),1.57(m,4H,N( CH 2 CH 2 CH 2 CH 2 CH 2 ));LC-MS:C 31 h 36 ClN 5 o 2 [M+1] + 546.20
Embodiment 32
[0063] Example 32-Methoxy-7-chloro-10-(3',5'-bis 4''-methylhexahydropiperidinium-4'-hydroxyphenyl)aminobenzo[b] Preparation of -1,5-naphthyridine (compound PYR_99)
[0064] Except that 4-methylpiperidine was used instead of 3-methylpyrrole, the compound 2-methoxy-7-chloro-10-(3',5'-bis4"- Preparation of methylhexahydropiperidinium-4'-hydroxyphenyl)aminobenzo[b]-1,5-naphthyridine (compound PYR99), yield 60.4%, melting point 172-174°C 1 H-NMR ((CD 3 ) 2 SO)δ:9.05(s,1H,Ar-NH),8.21-6.71(m,7H,Ar-H),4.04(s,3H,OCH 3 ), 3.36 (s, 4H, Ar- CH 2 N),2.86(m,8H,N( CH 2 CH 2 CHCH 2 CH 2 )CH 3 ),1.94(m,8H,N(CH 2 CH 2 CH CH 2 CH 2 )CH 3 ),1.60(m,2H,N ( CH 2 CH 2 CH CH 2 CH 2 )CH 3 ),1.00(d,6H,N(CH 2 CH 2 CHCH 2 CH 2 )CH 3 );LC-MS:C 33 h 40 ClN 5 o 2 [M+1] + 574.15
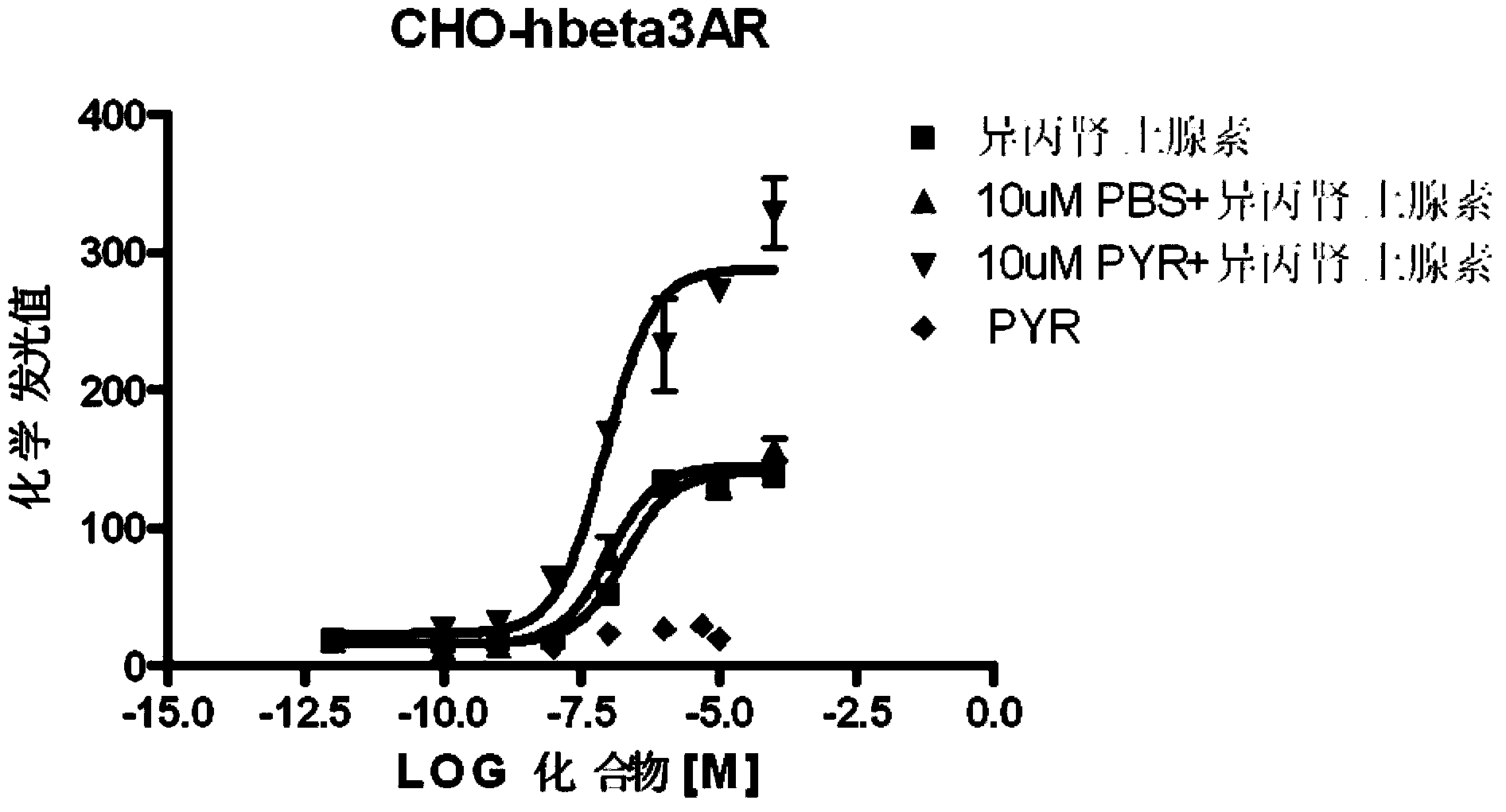
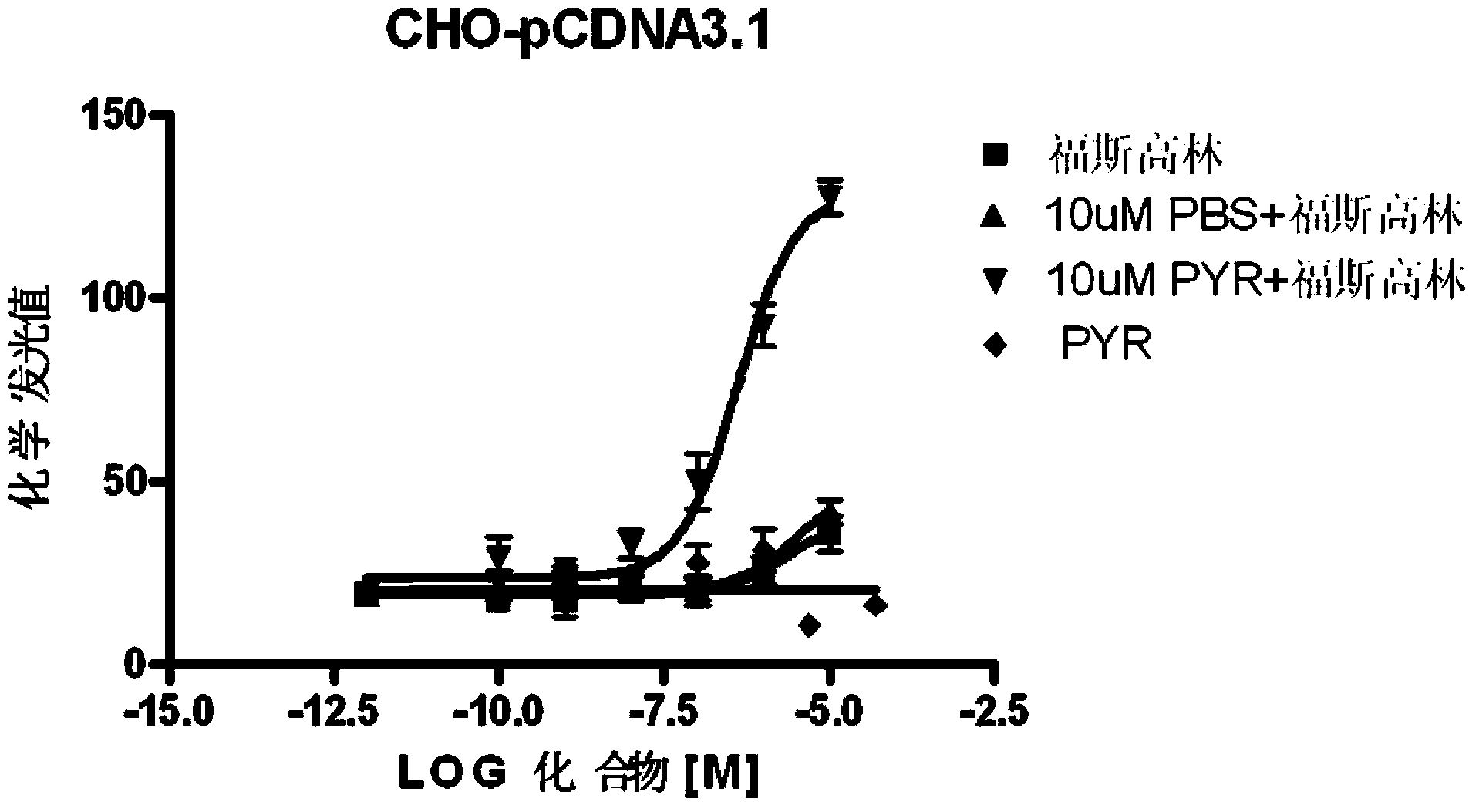
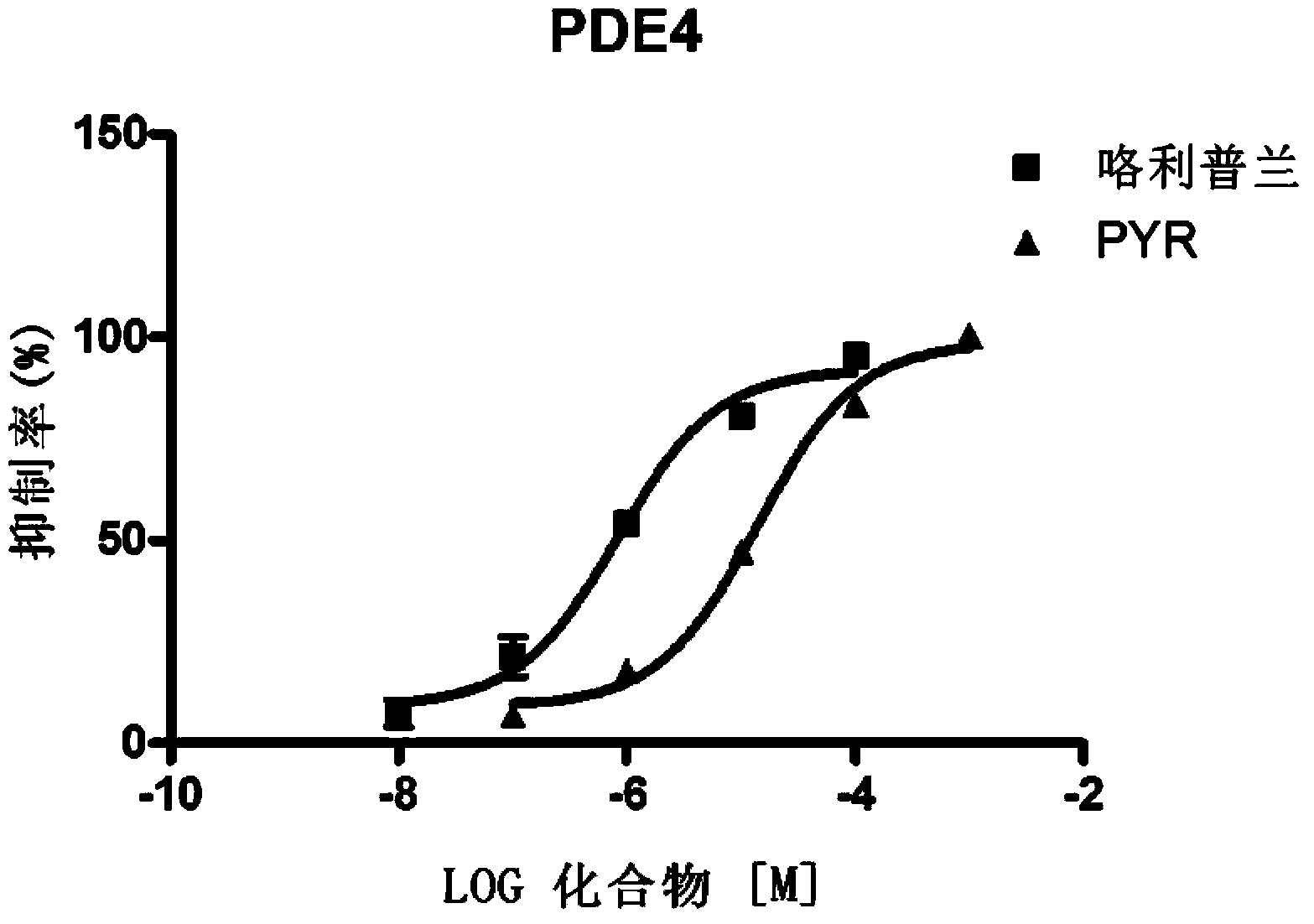
[0065] Examples of biological activity testing experiments:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com