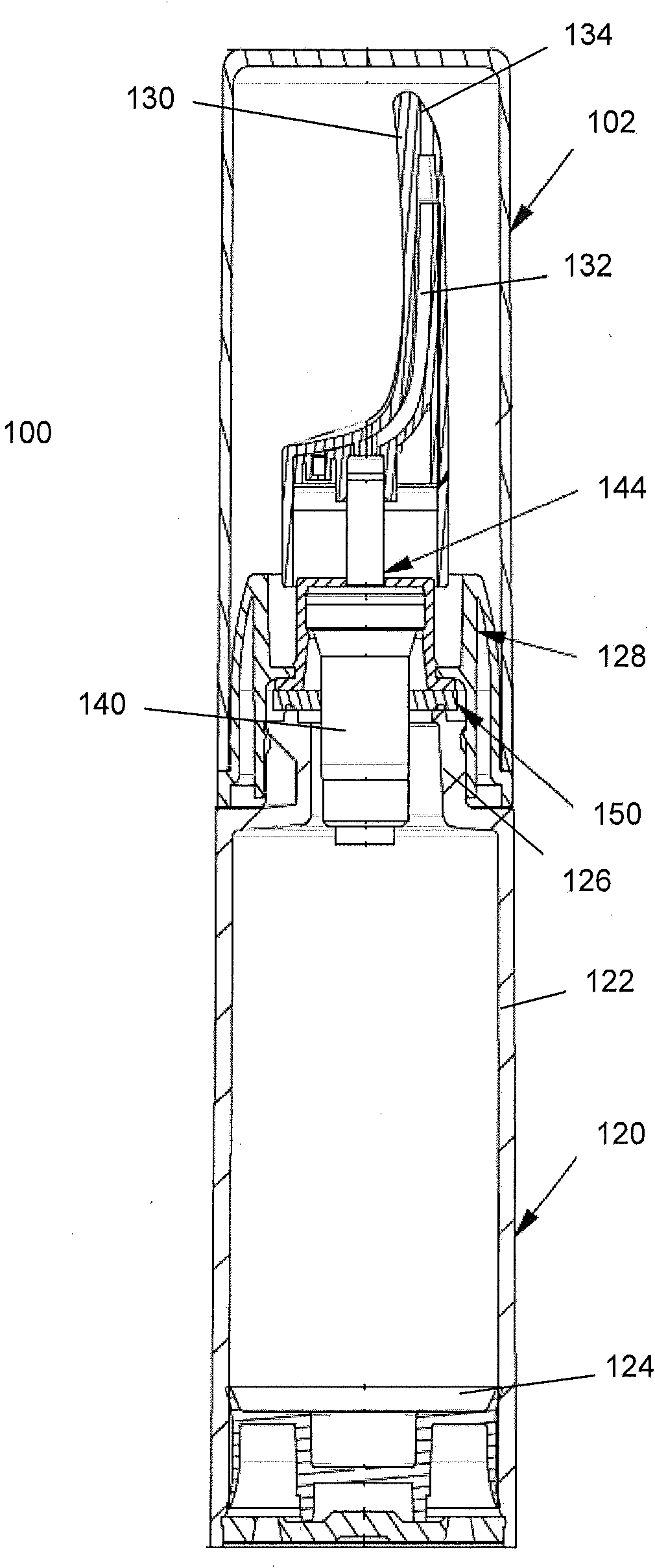
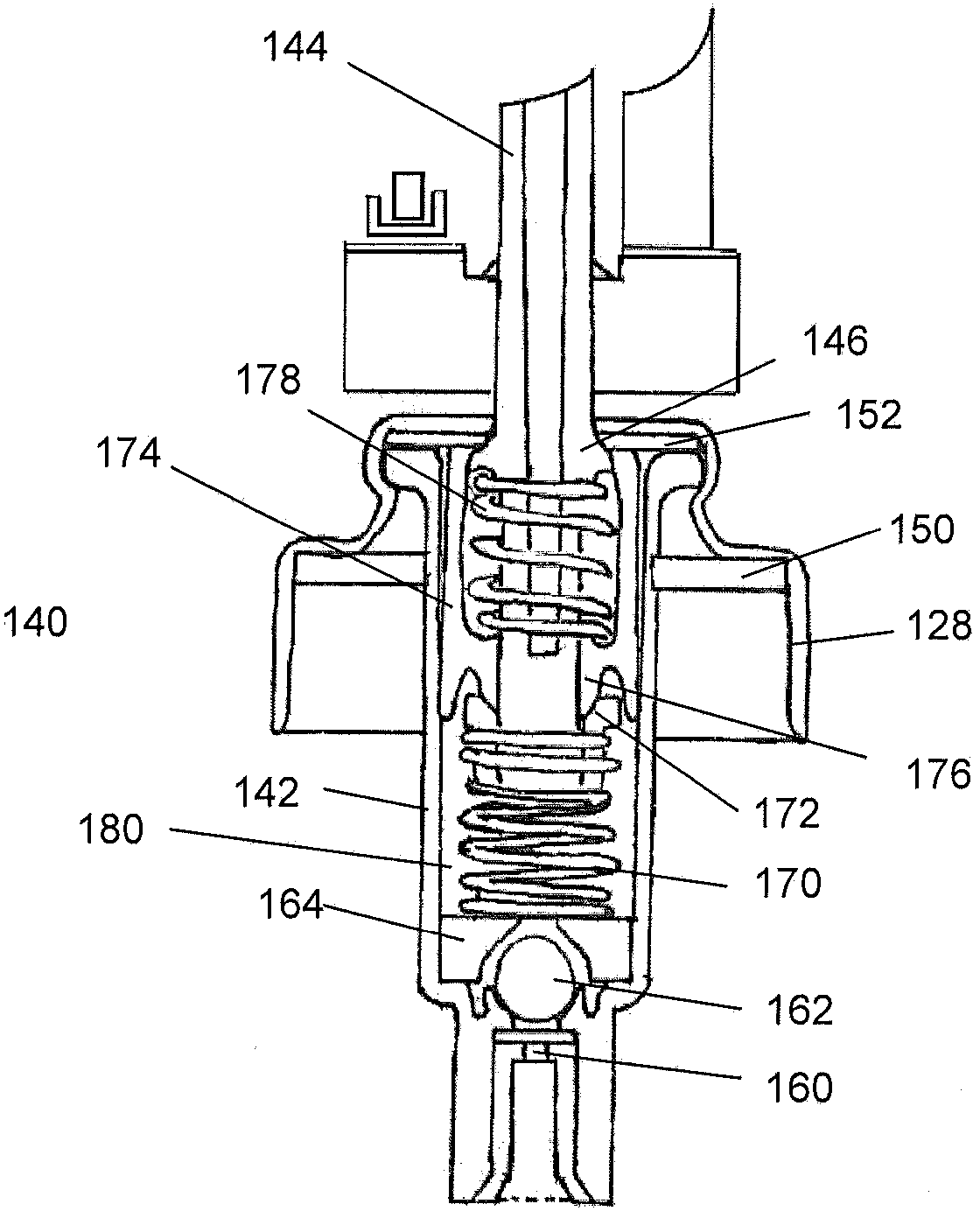
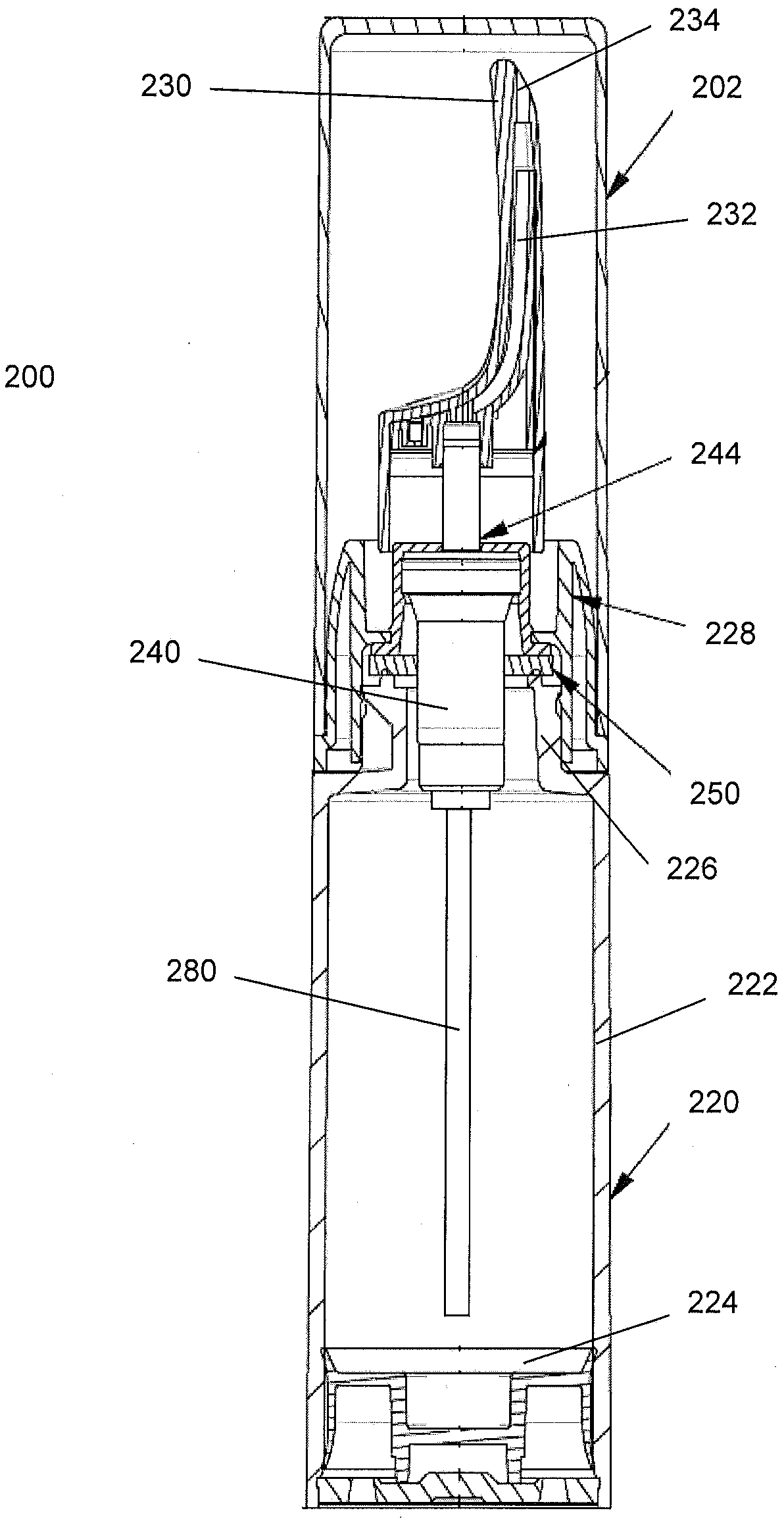
Controlled release nasal testosterone gels, methods and pre-filled multi-dose applicator systems for pernasal administration
A testosterone, gel technology, applied in the field of controlled-release nasal testosterone gel, for nasal administration and pre-filled multi-dose applicator system, can solve the problem of high testosterone protein binding, unnecessary, short hormone elimination half-life and other problems, to achieve the effect of good tolerance, safe use and easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0119] The preparation of intranasal testosterone gel is carried out by thickening oil mixture and blow-fill-seal technology packaging.
[0120] Introduce a lipophilic vehicle, e.g., castor oil, and a supersolvent, e.g., DMI, (step 1) into a mixing vessel (e.g., FrymaKoruma Vacuum-Mixing, Dispersing and Homogenising machine type Dinex700). The support and supersolvent mixture is blanketed with nitrogen (step 2) to vent oxygen and preheated, eg, to about 40°C to about 50°C.
[0121] The sieved (mesh size about 2 mm) testosterone (step 3) was added to the vehicle and supersolvent mixture and processed to obtain the following intermediate products.
[0122] Controlling the temperature of the vehicle and supersolvent mixture is necessary in the following steps to prevent a significant increase in temperature due to the shear strength applied to dissolve the testosterone.
[0123]
[0124] Homogenization of the intermediate product is carried out by controlling the temperature ...
preparation Embodiment
[0164] Examples of testosterone gels of the present invention are exemplified in Tables 1-3 below.
[0165] Table 1
[0166]
[0167] * Applied micronization
[0168] Table 2
[0169]
[0170]
[0171] *Preferably applied micronization
[0172] table 3
[0173]
[0174] *Preferably applied micronization
[0175] Intranasal testosterone gel formulations 14-21 are further examples (per 100 parts) of gel formulations contemplated by the present invention. Testosterone in micronized form is preferred.
[0176] gel 14
[0177]
[0178] gel 15
[0179]
[0180] gel 16
[0181]
[0182] gel 17
[0183]
[0184] gel 18
[0185]
[0186] gel 19
[0187]
[0188] gel 20
[0189]
[0190] gel 21
[0191]
[0192] Italicized is "surfactant"
[0193] gel 22
[0194]
[0195] gel 23
[0196]
[0197] gel 24
[0198]
[0199] gel 25
[0200]
[0201] gel 26
[0202]
[0203] gel 27
[0204]
[0205] gel 2...
Embodiment 2
[0219] Two dosage levels of an intranasal testosterone gel formulation, Trimel, in healthy adult male subjects Biopharma, Inc., Compleo of Canada TM Bid opening, balanced, random selection, crossover, double group, Dual therapy (dose levels 1 and 2), two-phase pharmacokinetic study
[0220] Test product for: Testosterone gel for nasal administration.
[0221] Profile Level 1:
[0222] Syringes, prefilled with 4.5% testosterone gel, delivering 6.75 mg of testosterone per nostril (manufactured by Trimel Biopharma, Inc. Canada). The formulation is as follows:
[0223] 4.5% Testosterone
[0224] 4% M1944
[0225] 3% (SiO 2 )
[0226] 88.5% castor oil.
[0227] Profile Level 2:
[0228] Compleo TM Syringe, prefilled with 6.5% testosterone gel, delivering 9.75 mg of testosterone per nostril (manufactured by Trimel Biopharma, Inc. Canada), based on 150 mg of Compleo TM Gel prefill weight. Compleo TM The gel formulation is as follows:
[0229]
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com