Novel peptides, compositions comprising the same and uses thereof in methods for the treatment of metabolic, cardiac and immune-related disorders
A technology for immune-related diseases and compositions, applied in the field of novel peptides, can solve problems such as insulin that has not progressed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0254] Isolation of APC-2 cDNA
[0255] Have the cDNA of the sequence shown in SEQ ID NO.2, its coding peptide APC-2 (have the sequence shown in SEQ ID NO.1), use the human cDNA library purchased from Clonetech and here as SEQ ID NO. The primers shown in 3 and 4 amplify it.
[0256]Regarding the APC-2 peptide, which is also named Uzi-1 herein, a maximum cleavage site likelihood of p=0.760 was determined between amino acid positions 17 and 18.
[0257] The PCR products were sequenced. PCR products were analyzed on agarose gels and stained using Cyber Green (Invitrogene), and the intensity of PCR products was assessed using a BioRad ChemiDoc analyzer. The results show that APC-2 is expressed in human heart and lymphocytes.
Embodiment 2
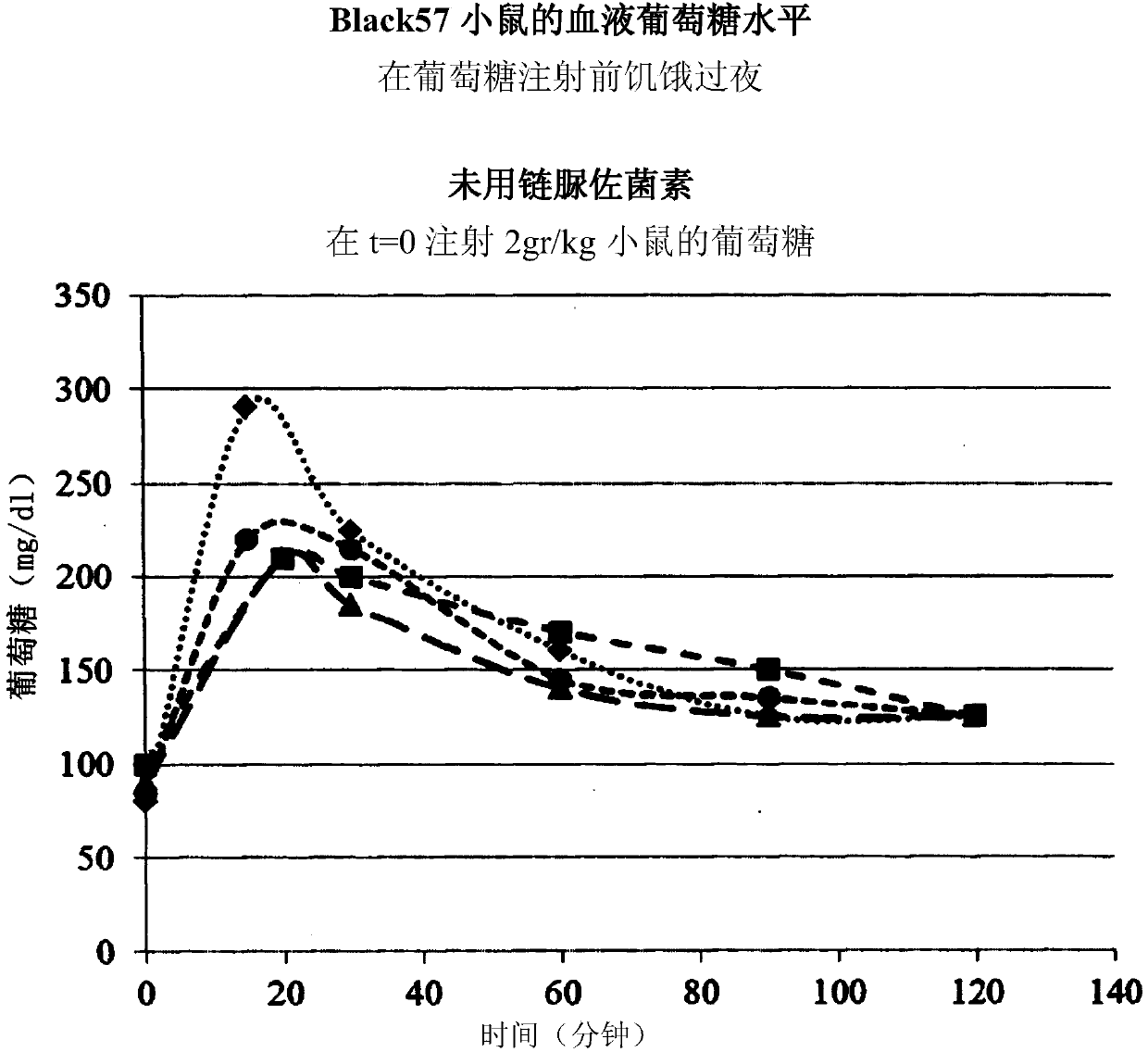
[0259] APC-2 improves glucose tolerance
[0260] Male C57Bl / 6 mice were divided into 4 groups of 5 mice each. The control group (Group I) received 5% mannitol IV (3 times a week, 200 μL each time). Group II received doses of APC2 (0.25 mg / Kg body weight) 3 times a week. Group III received doses of APC2 (1.5 mg / Kg body weight) 3 times a week. Group IV received doses of APC2 (15 mg / Kg body weight) 3 times a week. Mice were treated for 2 weeks.
[0261] At the end of the experiment, a glucose tolerance test (GTT) was administered as described in the methods section. exist figure 1 The results of GTT are shown in . It can be clearly seen that APC-2 improves glucose tolerance in a dose-dependent manner, with an optimal response observed for an APC2 dose of 1.5 mg / Kg body weight.
Embodiment 3
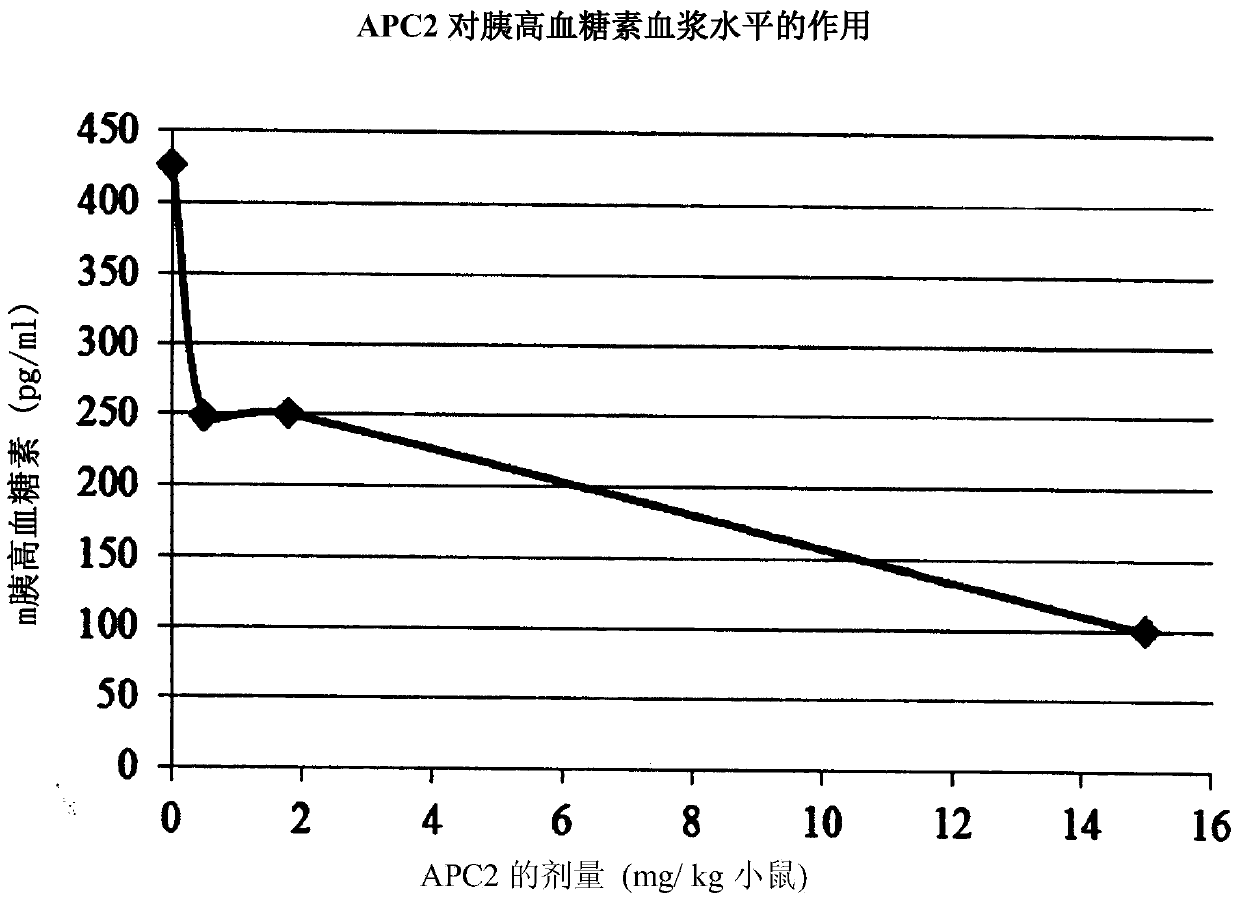
[0263] APC-2 reduces plasma glucagon
[0264] Mouse plasma glucagon levels were analyzed as described in the Methods section, except for the GTT. Such as figure 2 As shown, plasma glucagon levels were reduced in a dose-dependent manner by APC-2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com