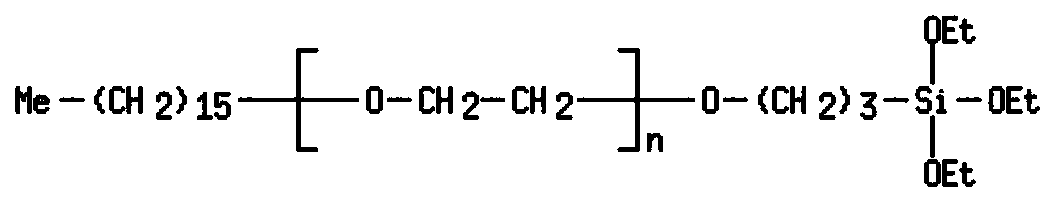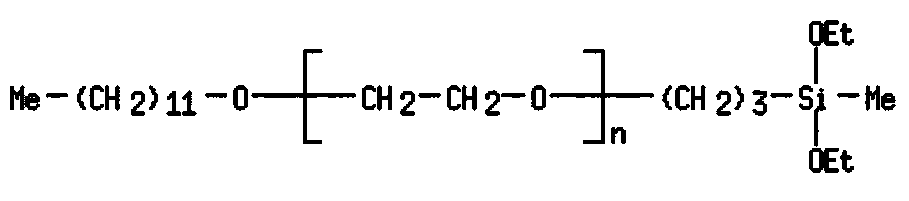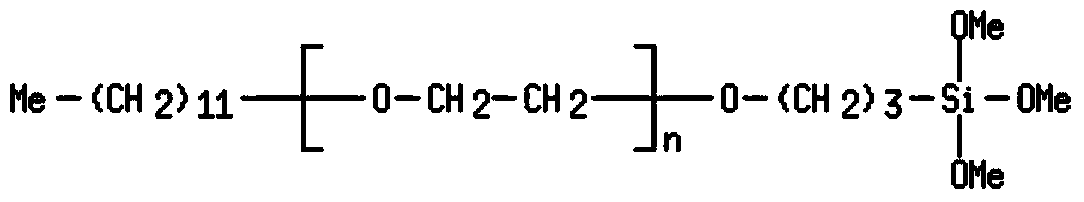Organosilicon compounds and the use thereof for producing hydrophilic surfaces
A technology for compounds and cross-linked compositions, applied in the fields of materials, formulations, and production of hydrophilic surfaces, can solve the problems of by-products that must be removed, unavailable, expensive feedstocks, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Production (Product 1)
[0135] (CH 3 O) 3 Si-CH 2 CH 2 CH 2 OCH 2 CH(OH)CH 2 OC(=O)CH 2 -O(CH 2 CH 2 O) 2-6 -(CH 2 ) 11-13 CH 3 with
[0136] (CH 3 O) 3 Si-CH 2 CH 2 CH 2 OCH 2 CH(CH 2 OH)OC(=O)CH 2 -O(CH 2 CH 2 O) 2-6 -(CH 2 ) 11-13 CH 3
[0137] In a glass flask with a Lebig condenser and receiver, 100 g of glycolic acid ethoxylated lauryl ether (Mn=460 g / mol) (as AKYPO RLM 45CA from KAO Chemicals GmbH, Emmerich, Germany) Obtained) Stir at 100°C and 20 mbar until no further separation of water occurs. After the nitrogen gas was introduced, 47g of (3-glycidoxypropyl)trimethoxysilane (in the trade name GF 80, commercially available from Wacker Chemie AG, Munich, Germany) and 0.5 g of 1,4-diazabicyclo[2.2.2]octane (as Commercially available from Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), and it was stirred at 100°C for two hours. Cooling and filtering yielded 135 g of slightly yellow liquid. 13 C NMR and titration showed more than 80% adduct formation.
[0138] 1g of...
Embodiment 2
[0140] Production (Product 2)
[0141] (EtO) 3 Si-CH 2 CH 2 CH 2 OCH 2 CH(OH)CH 2 OC(=O)CH 2 -O(CH 2 CH 2 O) 5-9 -Iso-C 13 H 27 with
[0142] (EtO) 3 Si-CH 2 CH 2 CH 2 OCH 2 CH(CH 2 OH)OC(=O)CH 2 -O(CH 2 CH 2 O) 5-9 -Iso-C 13 H 27
[0143] In a glass flask with a Lebig condenser and receiver, 100 g of glycolic acid ethoxy isotridecyl ether (Mn=570 g / mol) (as Marlowet 4538, available from Sasol Germany GmbH, Hamburg, Germany) Commercially available) Stir at 100°C and 20 mbar until no further water separation occurs. After the nitrogen gas was introduced, 45g of (3-glycidoxypropyl)trimethoxysilane (in the trade name GF 82, commercially available from Wacker Chemie AG, Munich, Germany) and 0.5 g of 1,4-diazabicyclo[2.2.2]octane (as Commercially available from Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), and it was stirred at 100°C for two hours. Cooling and filtering yielded 137 g of slightly yellow liquid. 13 C NMR and titration showed more than 80% adduct formation.
[0144] ...
Embodiment 3
[0146] Production (Product 3)
[0147] (EtO) 3 Si-CH 2 CH 2 CH 2 OCH 2 CH(OH)CH 2 OC(=O)CH 2 -O(CH 2 CH 2 O) 3-7 -(CH 2 ) 8 CH=CH(CH 2 ) 5-7 CH 3 ,
[0148] with
[0149] (CH 3 O) 3 Si-CH 2 CH 2 CH 2 OCH 2 CH(CH 2 (OH))OC(=O)CH 2 -O(CH 2 CH 2 O) 3- 7 -(CH 2 ) 8 CH=CH(CH 2 ) 5-7 CH 3
[0150] In a glass flask with a Lebig condenser and receiver, 105 g of glycolic acid ethoxy oleyl ether (Mn=540 g / mol) (as AKYPO RO 50VG, available from KAO Chemicals GmbH, Emmerich, Germany) Commercially available) Stir at 100°C and 20 mbar until no further water separation occurs. After the nitrogen gas was introduced, 43g of (3-glycidoxypropyl)trimethoxysilane (in the trade name GF 80, commercially available from Wacker Chemie AG, Munich, Germany) and 0.5 g of 1,4-diazabicyclo[2.2.2]octane (as Commercially available from Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), and it was stirred at 100°C for two hours. Cooling and filtering produced 138 g of a cloudy and yellowish liquid. 13 C NMR and ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com