Red phosphorescent iridium complex and preparation method thereof, and organic electroluminescence device
An iridium metal complex, red phosphorescence technology, applied in the fields of electric solid devices, organic chemistry, luminescent materials, etc., can solve the problems of backwardness and rarely achieve the color purity of dark red and dark green light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
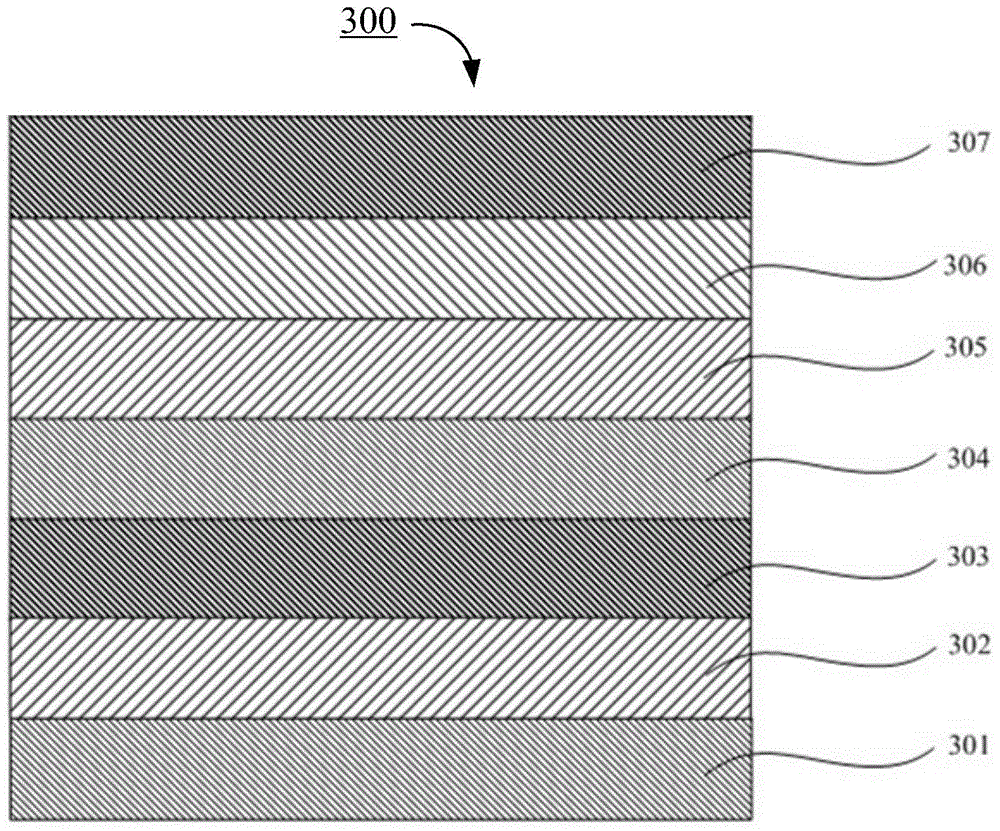
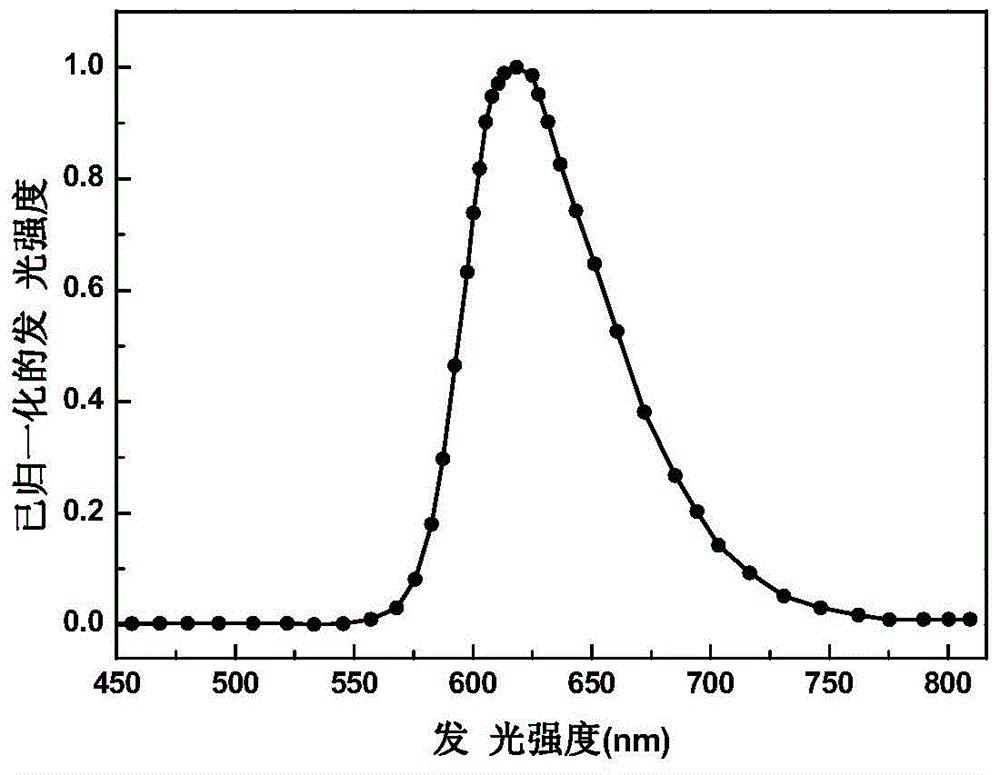
Image
Examples
preparation example Construction
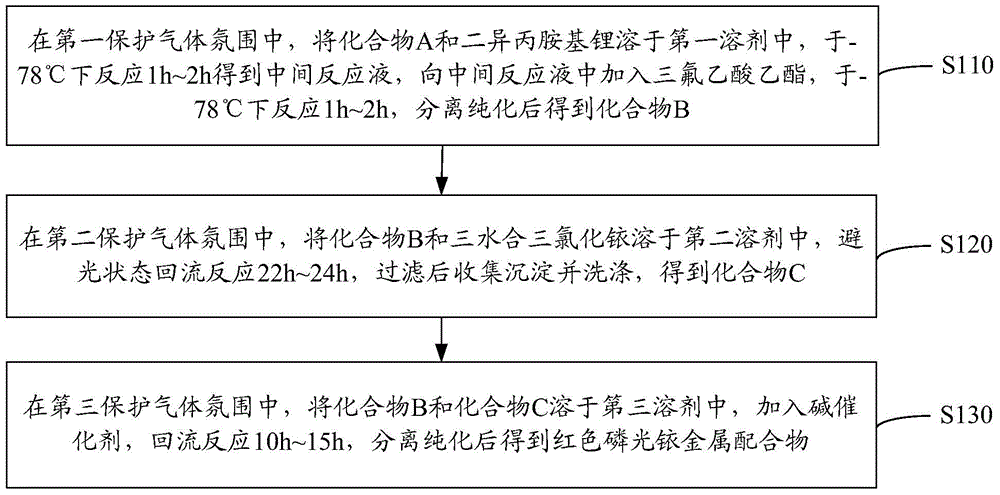
[0043] see figure 1 , a preparation method of a red phosphorescent iridium metal complex, comprising the following steps.
[0044] S110: In the first protective gas atmosphere, dissolve compound A and lithium diisopropylamide in the first solvent, react at -78°C for 1h to 2h to obtain an intermediate reaction liquid, and add ethyl trifluoroacetate to the intermediate reaction liquid The ester was reacted at -78°C for 1h to 2h, and compound B was obtained after separation and purification.
[0045] Compound A and lithium diisopropylamide ( ) in a molar ratio of 1:1.1 to 1.3, and the molar ratio of ethyl trifluoroacetate to lithium diisopropylamide is 1:1.
[0046] The structural formula of compound A is: The structural formula of compound B is: R is hydrogen or methyl. When R is a methyl group, R can replace the hydrogen on the 3-, 4-, 5-, 6-, 7- or 8-position of the isoquinoline ring. For example, compound B can specifically be 1-(4'-trifluoroacetylphenyl) isoquinolin...
Embodiment 1
[0083] Red Phosphorescent Iridium Metal Complex Tris[1-(4'-trifluoroacetylphenyl)isoquinoline-N,C 2 '] Synthesis of iridium.
[0084] (1) Synthesis of ligand 1-(4'-trifluoroacetylphenyl)isoquinoline.
[0085]
[0086] At -78°C, in a nitrogen atmosphere, 5 mL of a tetrahydrofuran solution containing 2.84 g (10 mmol) of 1-(4'-bromophenyl) isoquinoline was added to 1.29 g (12 mmol) of lithium diisopropylamide and 10 mL of In the mixed solution composed of tetrahydrofuran, stir and react for 1 h to obtain an intermediate reaction solution. A mixture consisting of 1.43 mL (12 mmol) of ethyl trifluoroacetate and 5 mL of tetrahydrofuran was added dropwise to the intermediate reaction solution, and the reaction system was maintained at -78° C., and stirred for 1 h. After the reaction was completed, the reaction system was naturally raised to 0°C, stirred and reacted for 0.5 h, then an appropriate amount of saturated ammonium chloride aqueous solution was added, and extracted with...
Embodiment 2
[0108] Red Phosphorescent Iridium Metal Complex Tris[1-(4'-trifluoroacetylphenyl)-3-methylisoquinoline-N,C 2 '] Synthesis of iridium.
[0109] (1) Synthesis of ligand 1-(4'-trifluoroacetylphenyl)-3-methylisoquinoline
[0110]
[0111] At -78°C, under a nitrogen atmosphere, 5 mL of a tetrahydrofuran solution containing 2.98 g (10 mmol) of 1-(4'-bromophenyl) 3-methylisoquinoline was added to 1.29 g (12 mmol) of diisopropylamine Lithium base and 10mL of tetrahydrofuran in a mixed solution, stirred for 1 h to obtain an intermediate reaction solution. A mixture consisting of 1.43 mL (12 mmol) of ethyl trifluoroacetate and 5 mL of tetrahydrofuran was added dropwise to the intermediate reaction solution, and the reaction system was maintained at -78° C., and stirred for 1 h. After the reaction system naturally rose to 0°C, stirred and reacted for 0.5 h, an appropriate amount of saturated ammonium chloride aqueous solution was added, extracted with ethyl acetate, and the organic ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Maximum lumen efficiency | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com