Expression vector for animal cell as well as preparation method and application of expression vector
An expression vector, animal cell technology, applied in the field of genetic engineering, can solve the problem of low target protein expression, achieve the effect of increasing yield and reducing translation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
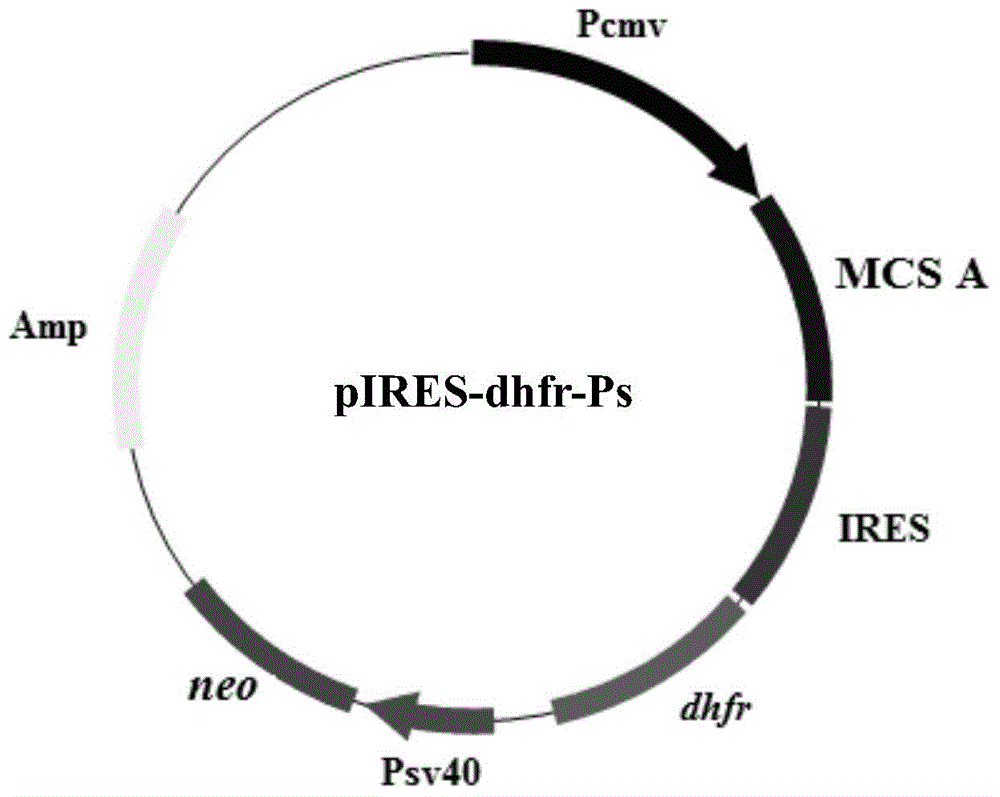
[0047] Embodiment 1 of the present invention provides an expression vector for animal cells, such as figure 1 As shown, the expression vector inserts the dhfr gene and the SV40-neo fragment into the pIRES vector digested by PvuII and BamHI, respectively, wherein the SV40-neo fragment is formed by connecting the SV40 early promoter gene and the neo gene, and the dhfr gene is located at Between the adjacent Xma I and Not I restriction sites; the SV40-neo fragment is located between the PvuII and BamHI restriction sites.
[0048] The above expression vector can be obtained by the following steps:
[0049] Step S101: using the pSV2-dhfr plasmid as a template and using SEQ ID No: 2 and SEQ ID No: 3 in the sequence table as primers for PCR amplification, wherein the primers shown in SEQ ID No: 2 contain an Xma I restriction site, The primer shown in SEQ ID No: 3 contains a Not I restriction site, and the pSV2-dhfr plasmid is from ATCC37146;
[0050] Step S102: The PCR product obta...
Embodiment 2
[0054] Embodiment 2 of the present invention provides an application of the expression vector of Embodiment 1, comprising the following steps:
[0055] Step S201: Insert the target gene into the multiple cloning site of the expression vector obtained in Example 1 to obtain a recombinant vector;
[0056] Step S202: using dhfr-deficient CHO cells as hosts to transfect expression vectors;
[0057] The day before transfection, inoculate 1X106 dhfr-deficient CHO cells in a 60mm culture dish, add 5ml serum-containing, antibiotic-free DMEM medium, 37°C, 5% CO 2 Culture until the cells are required to be 40%-80% confluent at the time of transfection. Dilute 5 μg of recombinant plasmid with OPTI-MEM1 medium to a total volume of 250 μl. After dilution, invert the centrifuge tube several times to mix the mixture. Add 10 μl transfection reagent LIPOFECTAMINE2000 to the mixture, and pipette several times with the pipette. Incubate the mixture at room temperature for 5-10 min. While inc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com