Preparation method and application of para-toluenesulfonate
A technology of methoxyphenyl and methyl, applied in the field of preparation and application of p-toluenesulfonate, can solve the problems of only 64% yield, increased production cost and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
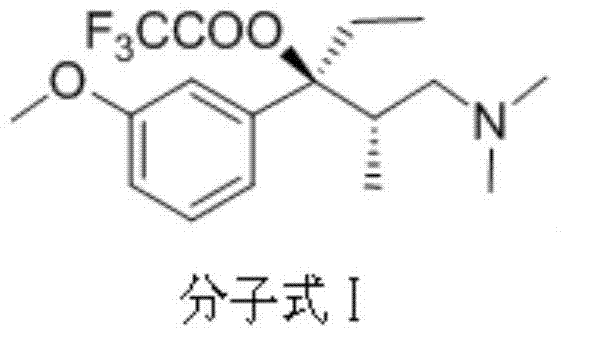
[0027] Example 1 Preparation of p-toluenesulfonate of (2S, 3R)-1-dimethylamino-3-(3-methoxyphenyl)-2-methyl-3-pentanol
[0028] Add 20g (2S, 3R)-1-dimethylamino-3-(3-methoxyphenyl)-2-methyl-3-pentanol to a 500ml round bottom flask, dissolve in 220ml tetrahydrofuran, and slowly Add 12.8g methanesulfonyl chloride and 16.6g triethylamine, after the reaction is complete, filter, add water, extract with dichloromethane, combine the organic layers, wash with water, wash with saturated brine, dry over magnesium sulfate, concentrate under reduced pressure and evaporate to dryness to obtain 27.1g of Yellow liquid, yield 98%.
Embodiment 2
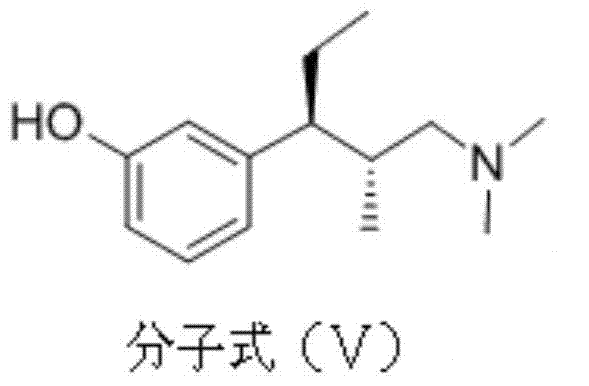
[0029] Example 2 Preparation of (2R, 3R)-3-(3-methoxyphenyl)-N,N,2-trimethylpentylamine
[0030] Add 20g (2S, 3R)-1-dimethylamino-3-(3-methoxyphenyl)-2-methyl-3-pentanol to a 500ml round bottom flask, dissolve in 220ml tetrahydrofuran, and slowly Add 12.8g methanesulfonyl chloride and 16.6g triethylamine, after the reaction is complete, add 0.7g of 10% palladium carbon, nitrogen replacement 3 times, hydrogen replacement 3 times, under 10 bar pressure, reaction temperature 45 ℃, hydrogenation reaction for 12 hours . Filter, concentrate under reduced pressure, adjust pH=9-10 with sodium carbonate aqueous solution, extract with dichloromethane, combine organic layers, wash with water, wash with saturated brine, dry over magnesium sulfate, and concentrate under reduced pressure to obtain 18.3 g of light yellow liquid, yield 90%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap