Two novel iridoid glycoside compounds, preparation method therefor and application thereof
A technology for iridoid glycosides and compounds is applied to two new iridoid glycoside compounds and their preparation fields, which can solve problems such as limiting wide application and achieve the effect of good medicinal prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
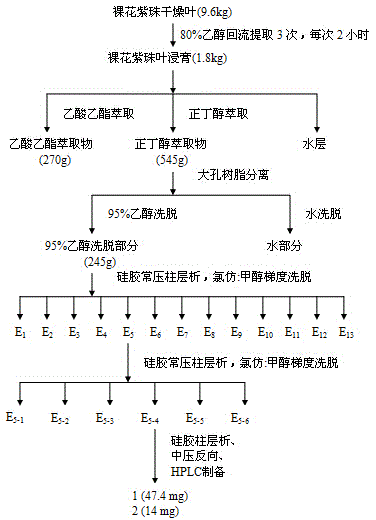
[0044] Example 1: Extraction and separation method of 7-O-E-p-coumaroyl-8-epi-carinaeic acid and 7-O-E-p-coumaroyl-garinia glycoside in the leaves of Aurantia japonica:
[0045] Take 9.6 kg of dried leaves of Aurora nudiflora, reflux extraction with 8 times the amount of 80% ethanol for 3 times, each time for 2 hours, combine the extracts, and use a rotary evaporator to recover the ethanol under reduced pressure under the condition of less than 50°C to obtain Extract 1.8kg; Suspend the extract in water to obtain an aqueous suspension of extract, extract the aqueous suspension of extract four times with ethyl acetate, then extract the aqueous extract of extract with n-butanol and mix it with ethyl acetate. Extract the extract water suspension after the suspension for three times, combine the extracted n-butanol layer liquid, recover n-butanol as a solvent for the n-butanol layer under reduced pressure at a temperature lower than 50°C, and obtain n-butanol layer immersion Cream ...
Embodiment 2
[0048] Example 2: Structural Identification of Two New Iridoid Glycosides in Auranthus nudica
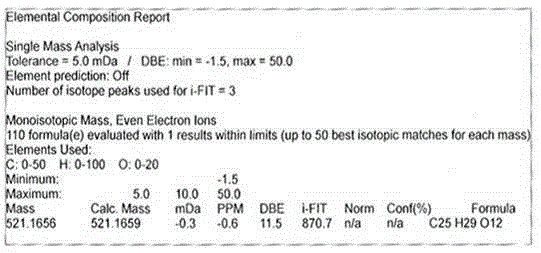
[0049] 1. 7-O-E-p-coumaroyl-8-epi-carpynic acid: white amorphous powder, easily soluble in organic reagents such as acetone, n-butanol, pyridine, methanol, etc., with a melting point of 179-180°C and optical activity [ α] 20 D -58.8 (c 0.08, MeOH); UV (CH 3 OH)λ max 288nm and 312nm, HRESIMS gives the molecular weight as m / z521.1656[M–H] – . Molecular formula is C 25 h 30 o 12 ; 1 H and 13 The C NMR data are shown in Table 1, and at the same time, by measuring the two-dimensional H-H correlation spectrum ( 1 H- 1 H COZY), H-C correlation spectrum (HSQC), H-C long-distance correlation spectrum (HMBC) and rotating coordinate system NOE (NOESY), the signal assignments of all carbon atoms and hydrogen atoms and the chemical structure of the compound were determined. The chemical structural formula is as follows:
[0050]
[0051] Table 1 The H and C spectra data of 7-O-E-...
Embodiment 3
[0060] Example 3: Anti-arachidonic acid-induced platelet aggregation activity test of two new iridoid glycoside compounds
[0061] Inhibition rate (%)=(absorbance value of monomer group-absorbance value of inducer group) / absorbance value of monomer group
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap