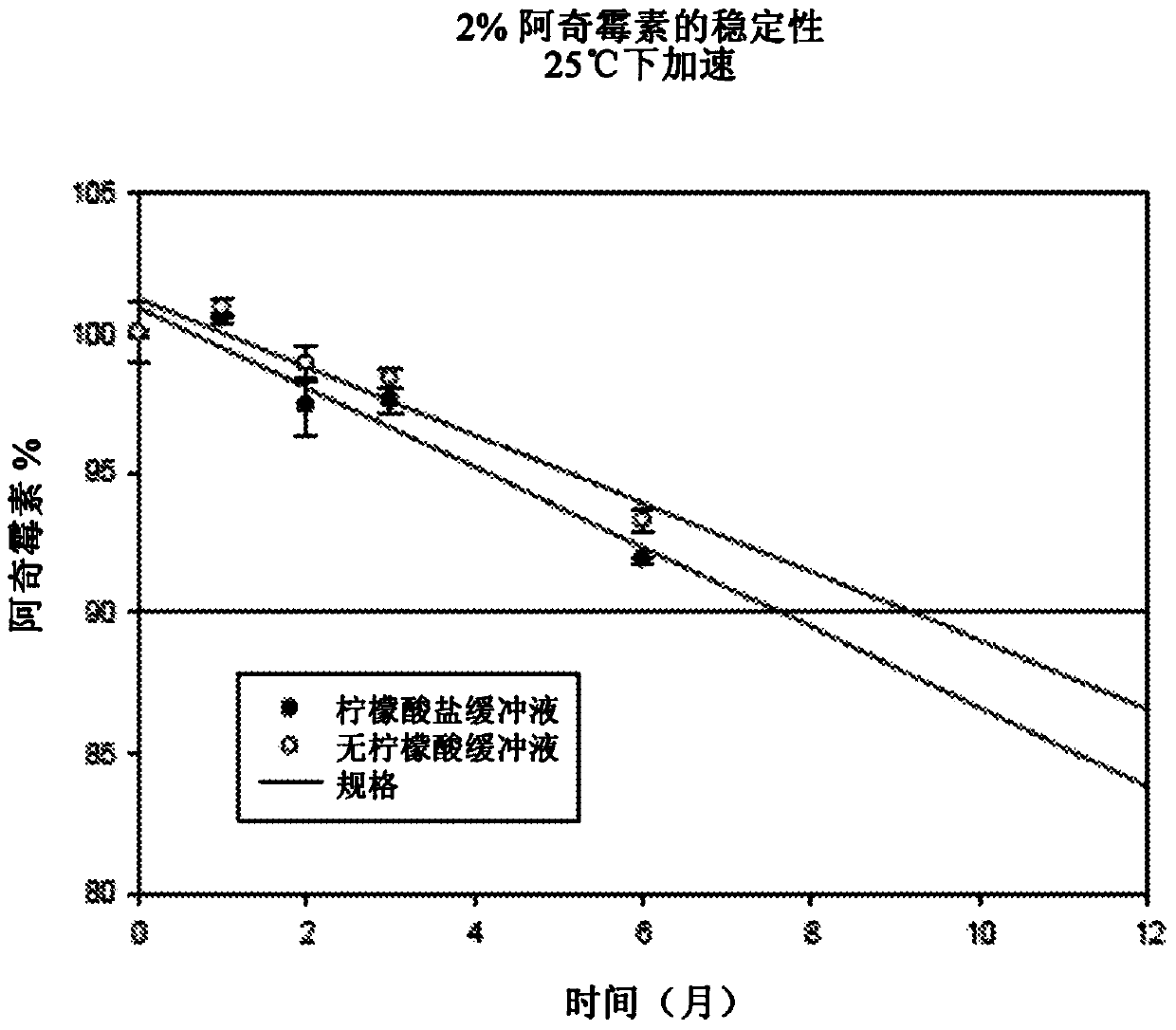
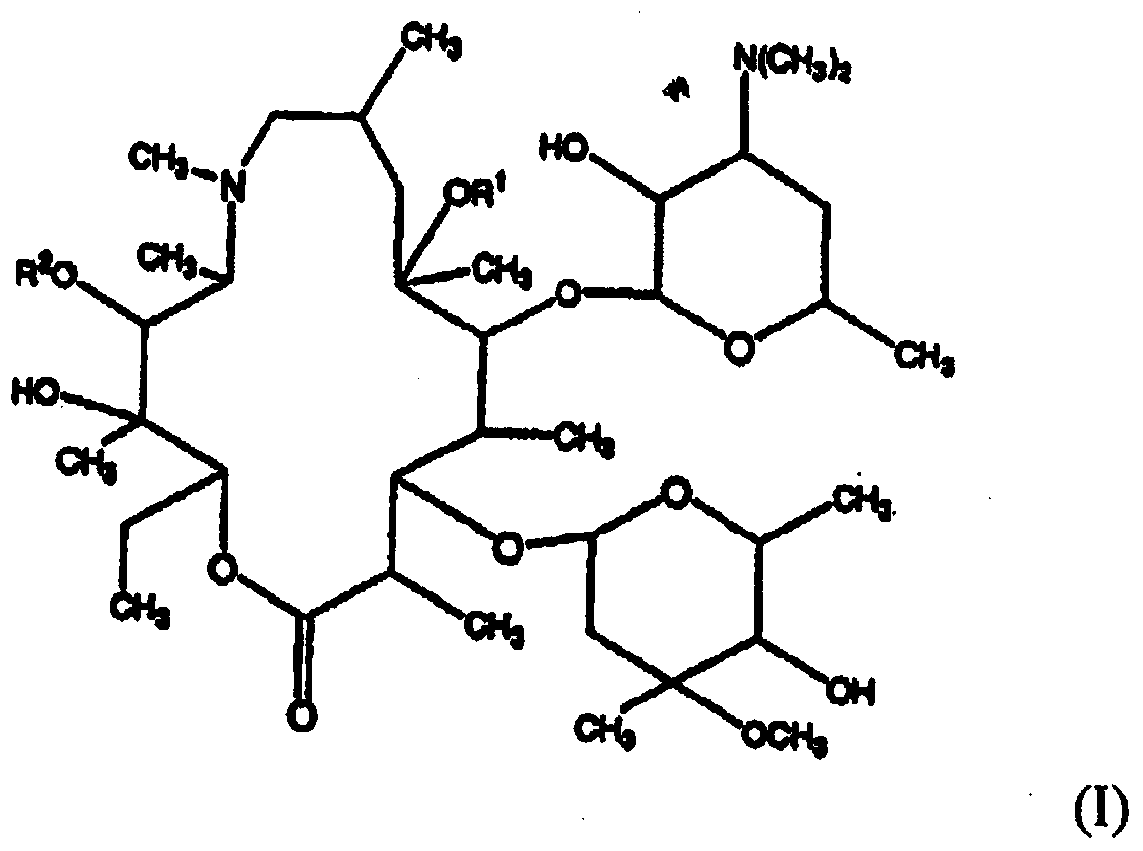
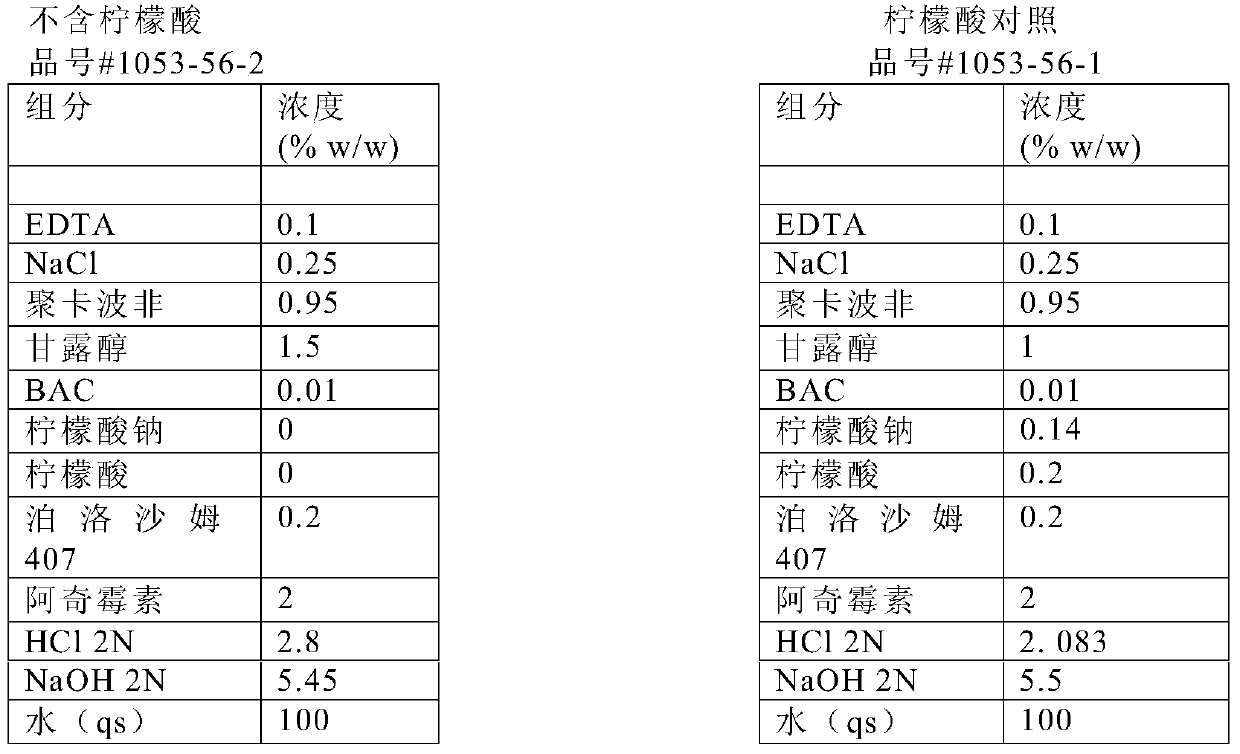
Concentrated aqueous formulation of azalides
A water-based preparation and preparation technology, applied in the direction of non-central analgesics, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Preparation of Ophthalmic Preparations in 12L Scale
[0071] In the first step, EDTA, sodium chloride, and polycarbophil (solution #1) were mixed for 30 minutes using an overhead mixer. The solution was then transferred to a stainless steel pressure tank. Use pressure to transfer the solution through a 100 mesh screen into a 12 L container. The solution was sterilized in the container at 121.1°C for 30 minutes. In the second step, sodium hydroxide was aseptically added to the container containing solution #1 through a 0.2 μm filter. The volume of sodium hydroxide required was based on making the final batch pH 6.3. The stainless steel pressure tank and transfer lines were then rinsed with house DI water. In the third step, mannitol, hydrochloric acid, azithromycin, benzalkonium chloride (BAC), and poloxamer 407 were mixed together (solution #2). Hydrochloric acid was added to facilitate the dissolution of azithromycin. After all solid ingredients in solution #2 we...
Embodiment 2
[0073] Embodiment 2 prepares the ophthalmic preparation of 12L scale with chitosan
[0074] In the first step, EDTA, sodium chloride, and polycarbophil (solution #1) were mixed for 30 minutes using an overhead mixer. The solution was then transferred to a stainless steel pressure tank. Use pressure to transfer the solution through a 100 mesh screen into a 12 L container. The solution was sterilized in the container at 121.1°C for 30 minutes. In the second step, sodium hydroxide was aseptically added to the container containing solution #1 through a 0.2 μm filter. The volume of sodium hydroxide required was based on making the final batch pH 6.3. The stainless steel pressure tank and transfer lines were then rinsed with residential deionized water and added to the vessel aseptically through a 0.2 μm filter. In the third step, mannitol and benzalkonium chloride are dissolved in water and added aseptically to the container. Disperse chitosan in water and add hydrochloric aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap