Novel 15-membered cyclic azalide, novel 16-membered cyclic diazalide derivative, and process for producing these
A compound, hydrogen atom technology, applied in the field of 15-membered ring azalide and new 16-membered ring diazalide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0127] The production method of the compound of the present invention is not limited to the method described above or the method specifically shown in the Examples. In addition, the compounds of the present invention are not limited to the compounds prepared by the methods described above or the methods specifically shown in the Examples, and compounds prepared by any method are included in the scope of the present invention. For example, compounds synthesized, produced, extracted, and purified by known methods according to the above general description and the specific description of the examples are of course also included in the present invention.
[0128] The compounds of the present invention form salts with many bases or acids, a property which can be exploited in the preparation of pure substances and as pharmaceutical delivery forms. That is, at the time of preparation, for example, it is made acidic, solubilized in a polar solvent such as water, extracted and refined,...
Embodiment 1
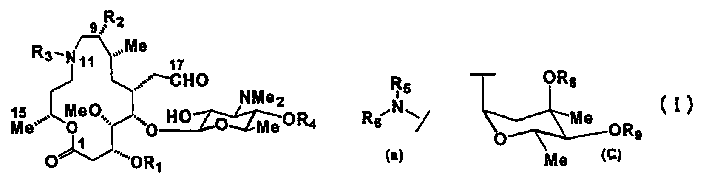
[0141] 9,2',3 "-tri-O-acetyl-10,11,12,13-tetrahydro-10,11,12,13-tetrahydroxymedecamycin 18-acetaldehyde dimethyl acetal (formula (VI ) shown in the compound, where R 1 is propionyl, R 2 is acetoxy, R 8 is acetyl, R 9 is propionyl, R 12 is acetyl, R 15 For the preparation method of methyl)
[0142] Add 610ml of acetonitrile to 64.2g of 9,3"-di-O-acetyl midecamycin 18-acetaldehyde dimethyl acetal (WO02 / 64607 bulletin), dissolve, add 7.8ml of acetic anhydride, stir at 40°C for 24 Hour.After the reaction solution was concentrated under reduced pressure, 660ml ethyl acetate was added, and the organic layer was washed 2 times with 300ml saturated aqueous sodium bicarbonate solution successively, and 300ml saturated brine was washed.After the organic layer was dried over anhydrous sodium sulfate, filter, and the filtrate was reduced Concentrate under reduced pressure, obtain 67.0g 9,2 ', 3 "-tri-O-acetyl midecamycin 18-acetaldehyde dimethyl acetal (compound shown in formula (V)...
Embodiment 2
[0150] (-)-(1R)-1-methyl-3-oxypropyl (3R, 4S, 5S, 6R, 8R, 9R)-9-acetoxy-5-[2-O-acetyl-4- O-(3-O-acetyl-2,6-dideoxy-3-C-methyl-4-O-propionyl-α-L-ribose-hexopyranosyl (hexopyranosyl))-3,6 -dideoxy-3-dimethylamino-β-D-glucopyranosyl]-6-(2,2-dimethoxyethyl)-4-methoxy-8-methyl-10-oxo -3-propionyloxydecanoate (compound shown in formula (VII), wherein R 1 is propionyl, R 2 is acetoxy, R 8 is acetyl, R 9 is propionyl, R 12 is acetyl, R 15 For the preparation method of methyl)
[0151] 30 mg of the compound of Example 1 was dissolved in 1 ml of benzene, and after adding 18 mg of sodium carbonate, 29 mg of lead tetraacetate was added in 5 portions over 20 minutes. After the reaction solution was stirred at room temperature for 1 hour, the supernatant was transferred to a separatory funnel. 5 ml of benzene was added to the residue, the supernatant was transferred to a separatory funnel, and the same operation was repeated three times. 10 ml of water and 15 ml of saturated aqueou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com