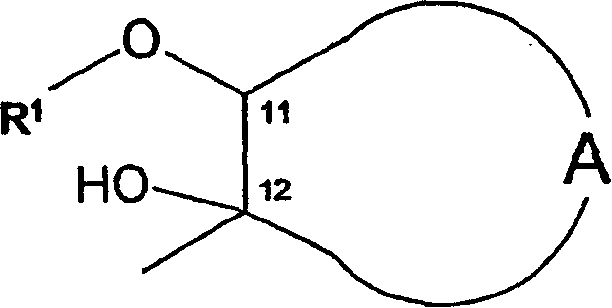
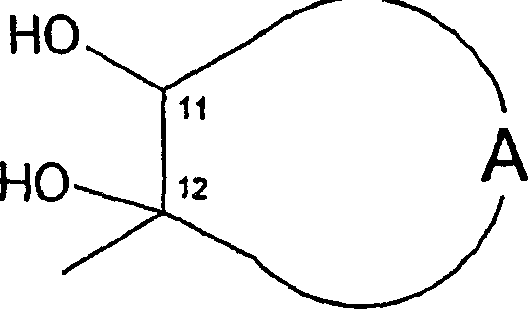
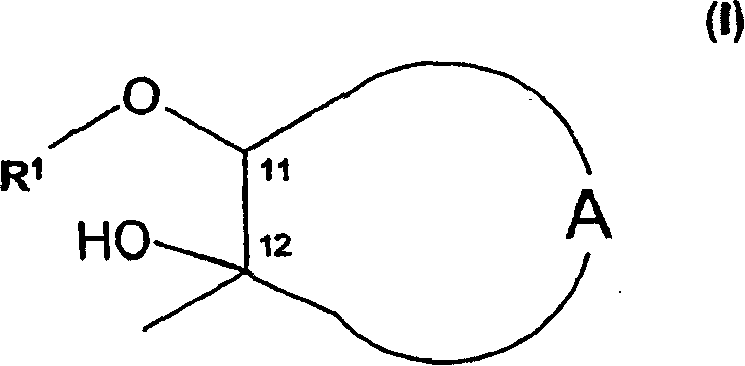
O-alkyl macrolide, azalide derivatives and their regioselective preparation process
A regioselective, azalide technology, used in sugar derivatives, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] 11-O-Methyl-Azithromycin
[0087] Method I
[0088] Azithromycin (75 g, 0.1 mol) and boric acid (3.1 g, 0.05 mol) were dissolved in absolute ethanol (300 mL), and then the yellow condensate of diazomethane (about 0.27 mol) obtained by method A was continuously added dropwise to the reaction in the mixture. The mixture was stirred at room temperature for 6 hours. A few drops of acetic acid were added to remove excess diazomethane. Ether was removed under reduced pressure, then ethanol was evaporated to a volume of 200 mL. The product was precipitated out by adding 400 mL of water. The crude product was dried in a vacuum oven at 40° C. for 12 hours. The yield was 87%. Crystallization of the product using ethanol / water afforded 100% pure (LC-MS analysis) 11-O-methyl-azithromycin in 73% yield.
[0089] ES-MS: m / z 763.2(M+H), 605.3(M+H-cladinose)
[0090] 1 H NMR (500MHz, CDCl 3 ): δ (ppm) 3.59 (s, 3H, 11-OMe), 3.42 (d, 1H, 11-H), 3.25 (dd, 1H, 2′-H), 3.03 (t, 1H...
Embodiment 2
[0098] 11-O-Methyl-2′-O,3′-N-Dibenzyloxycarbonyl-Azithromycin
[0099] 2'-O, 3'-N-dibenzyloxycarbonyl-azithromycin (J. Antibiotics 45 (1992) 527-534) (0.204g, 0.203mmol) and TiCl 4 (0.040g, 0.210mmol) was dissolved in DMF (5mL) and stirred at room temperature for 1 hour. A solution of diazomethane prepared by method B in diethyl ether (about 4 mmol) was added and the resulting mixture was stirred at room temperature for 6 hours. Use NaHCO 3 The mixture was diluted with aqueous solution (50 mL) and extracted with ethyl acetate (3 x 30 mL). Use Na 2 SO 4 The organic layer was dried and concentrated to obtain the title compound.
[0100] ES-MS: m / z 1017.3(M+H), 859.4(M+H-cladinose)
Embodiment 3
[0102] 11-O-Methyl-9-deoxy-9a-aza-9a-homoerythromycin
[0103] 9-deoxy-9a-aza-9a-homoerythromycin (J.Chem.Soc.Perkin Trans.1(1986)1881-1890) (1.00g, 1.36mmol) and H 3 BO 3 (0.084g, 1.36mmol) was dissolved in acetonitrile (20mL) and stirred at room temperature for 1 hour. A solution of diazomethane prepared by method B in diethyl ether (about 6 mmol) was added and the resulting mixture was stirred at room temperature for 2 hours. Use NaHCO 3 The mixture was diluted with aqueous solution (50 mL) and extracted with ethyl acetate (3 x 30 mL). Use Na 2 SO 4 The organic layer was dried and concentrated to obtain the target compound (0.813 g, yield 80%).
[0104] ES-MS: m / z 749.6(M+H), 591.5(M+H-cladinose)
[0105] 1 H NMR (500MHz, CDCl 3 ): δ (ppm) 3.56 (s, 3H, 11-OMe), 3.43 (d, 1H, 11-H), 3.30 (dd, 1H, 2′-H), 3.03 (t, 1H, 4″-H )
[0106] 13 C NMR (125MHz, CDCl 3 ): δ (ppm) 84.3 (11-C), 78.1 (4″-C), 70.9 (2′-C) 62.4 (11-OMe)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com