Screening method for therapeutic agents for Charcot-Marie-Tooth disease and self-differentiation motor neurons used therefor
A technique of motor neuron and screening method, applied in the field of preparation of induced pluripotent stem cells, can solve the problems of drug selection limitation and difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Isolation of CMT Patient-Derived Cells by Skin Biopsy
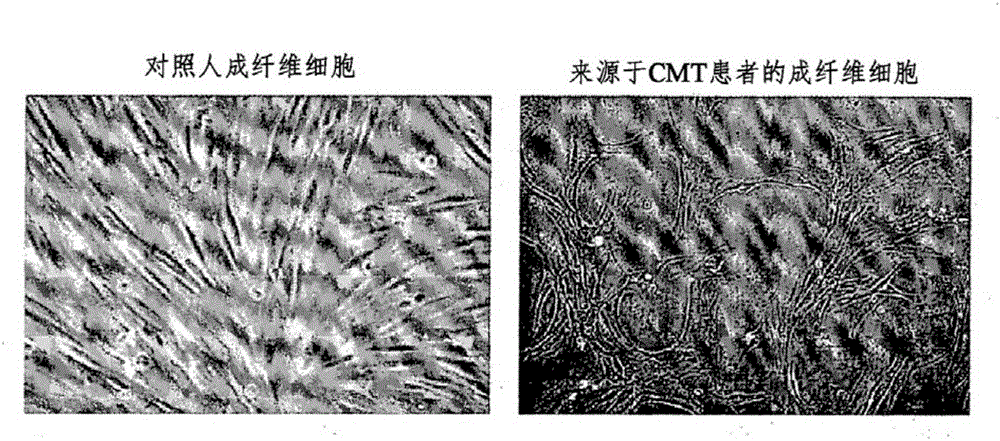
[0082] Skin biopsy is a safe, less invasive and economical method for pathological diagnosis of skin lesions. Under the approval of the institutional review board, the present inventors interviewed CMT2F patients exhibiting S135F or P182L mutations in the HSP27 gene as well as normal volunteers (Ewha Womans University Mokdong Hospital, Korea). To perform skin biopsy, local anesthesia was given to normal volunteers and CMT patients, and skin biopsy was performed by using a punch with a 4 mm diameter roundblade. Skin tissue obtained by skin biopsy was loaded in DMEM supplemented with: 10 mg / ml type IV collagenase (Invitrogen, USA), 50 U / ml dispase (Roche) and 0.05% trypsin / EDTA, followed by incubation at 37° C. The reaction was carried out for 40 minutes. The resulting cell suspension was filtered through a nylon cell strainer (passing particles up to 70 [mu]m in size). The resulting fibroblasts were cu...
Embodiment 2
[0087] Example 2: Preparation of induced pluripotent stem cells (iPSC) and embryoid bodies derived from CMT patients
[0088] Inducing the development of iPSCs derived from CMT patients
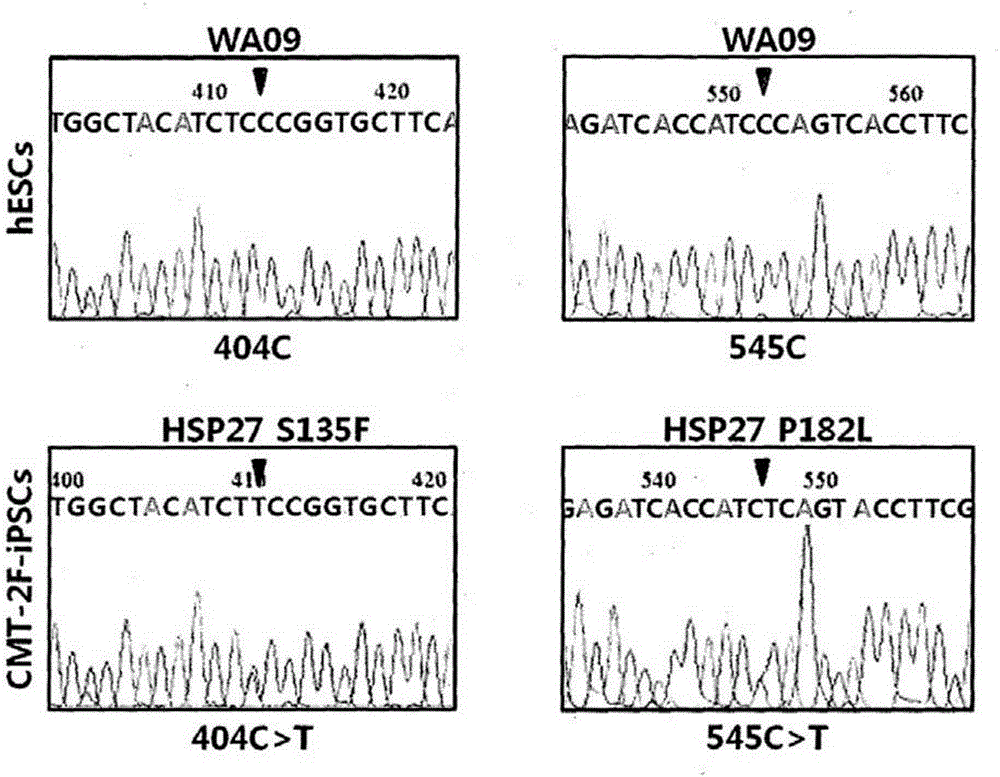
[0089] To prepare iPSCs for neuronal differentiation from fibroblasts obtained from CMT patients by skin biopsy in Example 1, Sendai protein containing 4 transcription factors (Klf4, Oct3 / 4, Sox2, and c-Myc) was used. Virus system (CellBiolabs, USA) was used to transfect fibroblasts from normal control group and CMT patients. The Sendai virus used did not insert into the host genome, instead it disappeared after several subcultures, suggesting that more stable iPSCs were obtained. The dose of Sendai virus was determined as MOI (multiplicity of infection)3. Cells were infected with Sendai virus overnight, and then the medium was replaced with DMEM supplemented with 10% FBS, followed by further culture for 6 days to stabilize the cells. Then, the cells were transferred to SNL feeder cells (...
Embodiment 3
[0111] Example 3: Inducing the differentiation of motor neurons derived from CMT patients and their differentiation efficiency
[0112] Differentiation of motor neurons from CMT2F-iPSCs
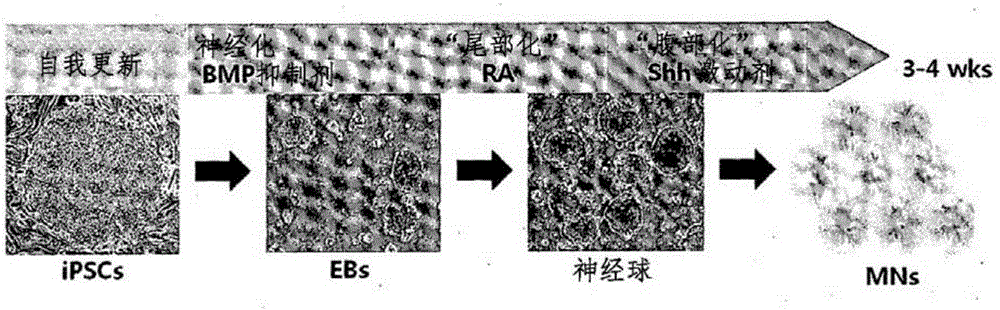
[0113] In order to use CMT2F-iPSCs as a peripheral neuropathy model, the figure 2 Differentiation from CMT2F-iPSCs to motor neurons was induced in the same manner as described in (AmorosoMW et al., JNeurosci2013;33:574-586)( figure 2 ).
[0114] Specifically, in order to induce embryoid body formation, CMT2F-iPSCs (S135F and P182L) or normal control WA09_hESCs induced in the same manner as described in Example 2 were divided into small clumps, and then supplemented with Suspension culture in ESC / iPSC medium (basic medium) for 2 days: 10 μM Y27632 (Rho-related kinase inhibitor, Tocris Bioscience, UK), 20 ng / ml bFGF (Invitrogen, USA), 10 μM SB435142 (Stemgent, USA), 0.2 μM LDN193189 (Stemgent , USA) and penicillin / streptomycin.
[0115] 3 days after the start of culture, the basal medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com