Uses of butylidenephthalide and method for preparing pharmaceutical composition from butylidenephthalide
A technology of butenylphthalide and pharmaceutical composition, which is applied in the field of application of butenylphthalide and its preparation as a pharmaceutical composition, can solve problems such as threats to human health, and achieve treatment or prevention of neurodegenerative diseases, The effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Synthesis of aqueous phase butenylphthalide
[0040] Butenyl phthalide (n-butylidenephthalide, Bdph, W333301) and F127 polymer substance (Pluronic F127, P2443) were obtained from Sigma-Aldrich, USA. After mixing 10 mg of butenylphthalide and F127 macromolecular substance at a weight ratio of 1:1 or 1:2, dissolve it in 2 ml of tetrahydrofuran (tetrahydrofuran), add it to 10 ml of water, and then heat rapidly The tetrahydrofuran was removed and freeze-dried. Afterwards, it is dissolved in water again, and the butenylphthalide particles emulsified in the solution have a size of about 30-200 nm and a polydispersity index of 0.2-0.5.
Embodiment 2
[0041] Example 2: Cell Culture
[0042] Human pluripotent stem cells were cultured in serum-free medium Essential8 TM (LifeTechnology company, USA), and with matrigel TM Matrigel (Becton-Dickinson Company, USA) was used for attachment culture. Aspirate the culture medium, wash the cells twice with phosphate buffer, then add the enzyme Accutase TM (MerckMillipore Company, USA) After reacting for 2 to 5 minutes, neutralize with culture medium, wash down the cells and break up into small clumps, centrifuge at 1000rpm for 2 minutes, then absorb the supernatant, and put them into a new culture dish Culture in medium for about 3 to 5 days, then subculture, and the culture medium must be changed every day during the culture period.
Embodiment 3
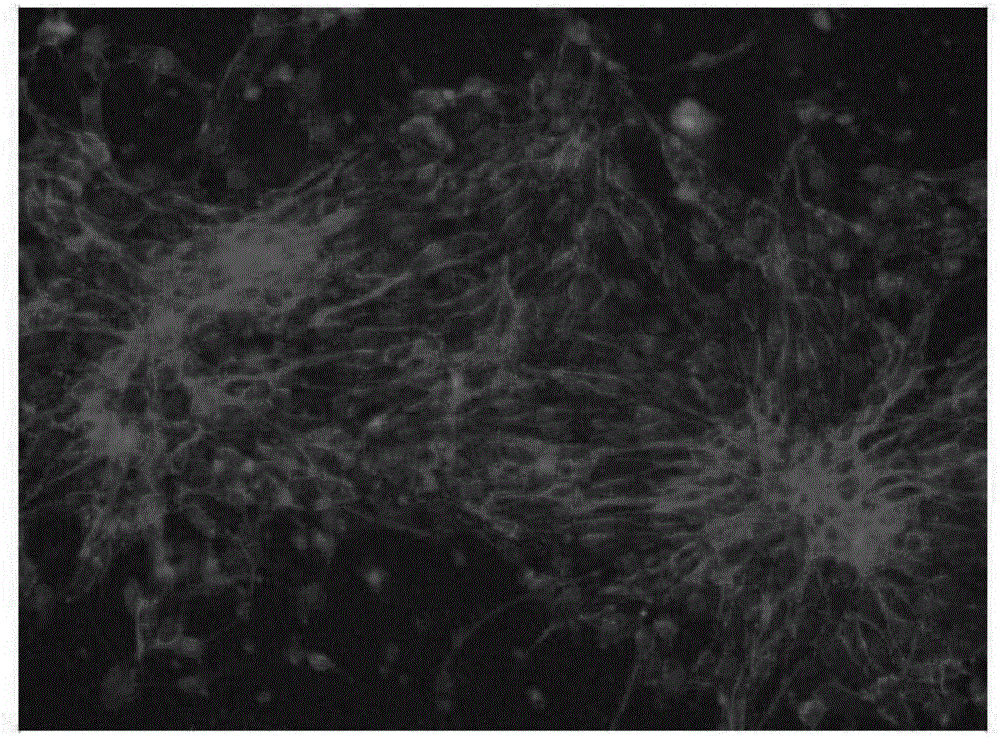
[0043] Example 3: Neural cell differentiation
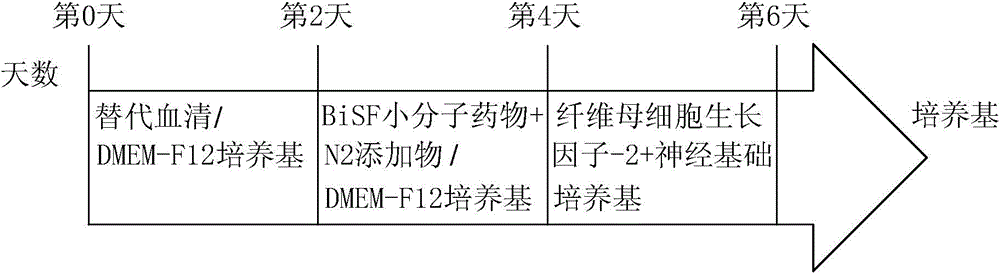
[0044] Culture the human pluripotent stem cells to 8-9 minutes full, wash the cells with phosphate buffered saline twice, and use the enzyme Accutase TM After acting for 2 to 5 minutes, add culture medium to dilute the enzyme, then wash the cells down, break up to an appropriate size, and centrifuge at 800rpm for about 2 minutes, and then place the cells in a place containing 20% serum replacement (knockoutserum replacement, KSR for short). , LifeTechnology Company, U.S.A.) DMEM-F12 medium, carried out suspension culture with uncovered petri dish for 2 days, and obtained embryoid body (Embryonicbody) suspension.
[0045] Put the suspended embryoid body in a centrifuge tube, after natural sedimentation, remove the supernatant, and carry out suspension culture for 2 days with the neural induction medium containing BiSF small molecule drug, and then, the suspended embryoid body will appear ring-shaped epithelial cells Structure, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com