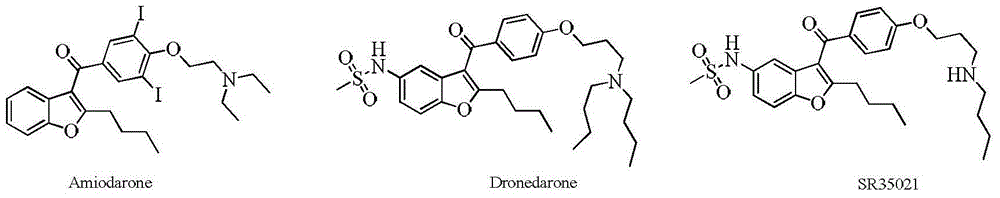
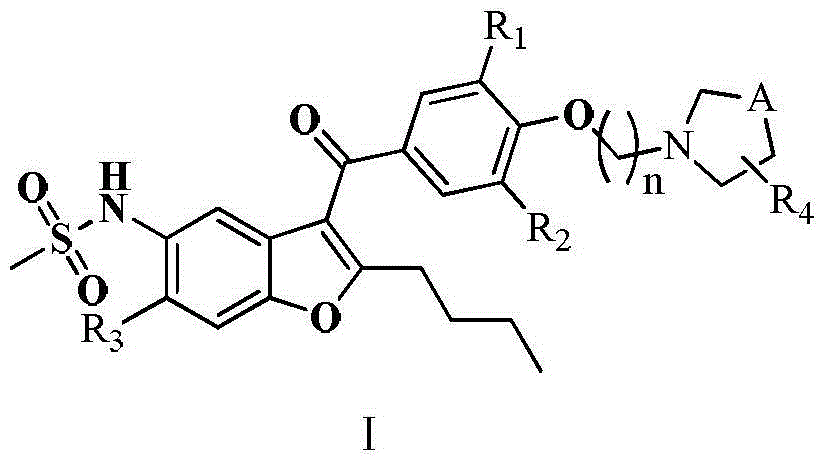
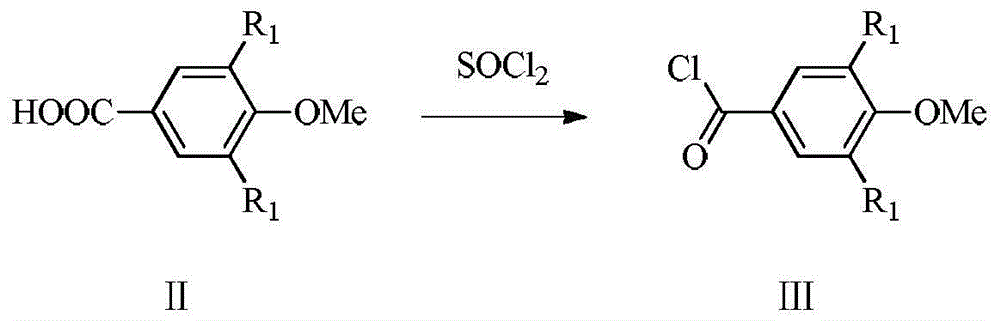Benzofuran derivative, preparation method and application thereof
A technology for benzofuran and derivatives, which is applied in the fields of preparing antiarrhythmic drugs and treating related diseases, and can solve the problems of poor water solubility, oral bioavailability of liver first-pass effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0077] Preparation Example 12-Butyl-3-[4-(3-chloropropoxy)benzoyl]-5-nitrobenzofuran
[0078] 2-Butyl-3-(4-hydroxybenzoyl)-5-nitrobenzofuran (33.94g, 100mmol) was added to 300ml of 95% acetonitrile aqueous solution, potassium carbonate (27.64g, 200mmol) and tetrabutyl Ammonium bromide (1.61g, 5mmol), heated to reflux for 20min, added 1-bromo-3-chloropropane (17.32g, 110mmol), refluxed for 17h, cooled the reaction solution to room temperature, filtered, and washed the filter cake with a small amount of acetonitrile, The filtrate was concentrated, and the residue was separated and purified by silica gel column (eluent, petroleum ether:ethyl acetate=10:1, v:v) to obtain 28.3 g of light yellow solid with a yield of 68%.
preparation Embodiment 2
[0079] Preparation Example 22-Butyl-3-[4-[3-(piperidin-1-yl)propoxy]benzoyl]-5-nitrobenzofuran
[0080] 2-Butyl-3-[4-(3-chloropropoxy)benzoyl]-5-nitrobenzofuran (8.32g, 20mmol) was dissolved in 60ml of acetonitrile, piperidine (2.56g, 30mmol), potassium carbonate (8.29g, 60mmol) and potassium iodide (0.33g, 2mmol), reflux reaction for 24h, the reaction solution was cooled to room temperature, filtered, the filter cake was washed with a small amount of acetonitrile, and the filtrate was concentrated. The residue was dissolved in 60m ethyl acetate, washed with saturated brine (50ml×2) and water (50ml×2), the organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to give 8.08g yellow oil, the yield was 87%.
preparation Embodiment 3
[0081] Preparation Example 32-Butyl-3-[4-[3-(piperidin-1-yl)propoxy]benzoyl]-5-aminobenzofuran
[0082] 2-Butyl-3-[4-[3-(piperidin-1-yl)propoxy]benzoyl]-5-nitrobenzofuran (4.65g, 10mmol) was dissolved in 45ml of absolute ethanol 0.93g of palladium carbon was added, the reaction system was filled with hydrogen three times, and reacted at 50°C for 16h under the condition of flowing hydrogen. The reaction solution was cooled to room temperature, filtered, the filter cake was washed with a small amount of absolute ethanol, and the filtrate was concentrated to obtain 4.15 g of a yellow oil with a yield of 95%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap