Ophthalmic composition, method for preparing the same, and use of the same
A technology of composition and ophthalmic medicine, which is applied in the field of ophthalmology, can solve the problem that it cannot be administered during the day
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0134] Preparation method of ophthalmic composition
[0135] In one embodiment, the preparation method of the ophthalmic composition of the present invention comprises;
[0136] 1) prepare lipid nanodispersion and gel matrix separately,
[0137] Wherein the preparation of the lipid nanodispersion comprises:
[0138] i) mixing an oil phase dispersion containing solid lipids, liquid lipids and an oil phase emulsifier dispersed in an organic solvent with water or an aqueous phase solution containing a water phase emulsifier dissolved in water to obtain a pre-emulsion (preemulsion), the temperature of the oil phase dispersion is preferably 60°C to 90°C, the temperature of the water or aqueous phase solution is preferably 60°C to 90°C, and the mixing time is preferably 3-10 minutes;
[0139] ii) homogenizing the pre-emulsion, the homogenization is preferably carried out in a pressure range of 500-1500 bar for 6-20 cycles;
[0140] iii) removing the organic solvent in the homog...
Embodiment 1
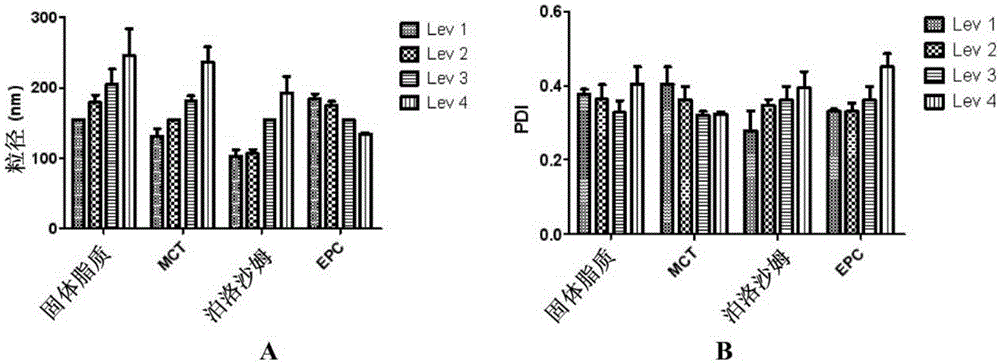
[0170] Embodiment 1: single factor experiment
[0171] To understand how ingredients affect the particle size and PDI of lipid nanodispersions, single factor experiments were performed. On the basis of basic formulations, solid lipids (yellow petrolatum: lanolin = 8:1, w / w), liquid lipids (MCT), surfactants (EPC, poloxamer 188 )concentration.
[0172] Preparation:
[0173] To prepare lipid nanodispersions, high-pressure homogenization was performed. First, solid lipid, liquid lipid (MCT) and EPC composed of yellow petrolatum and lanolin (8:1, w / w) were dissolved in 20 mL of ethanol at 75 °C as the oil phase. Then 100 mL of water (75° C.) containing 0.5% of poloxamer 188 was prepared as the aqueous phase. after using Under the condition of WERKE stirring at 1000rpm, slowly pour the oil phase into the water phase. After 5 minutes of continuous stirring a pre-emulsion was obtained. Nanoemulsions were then prepared by homogenizing the pre-emulsion using an AST homogenizer ...
Embodiment 2
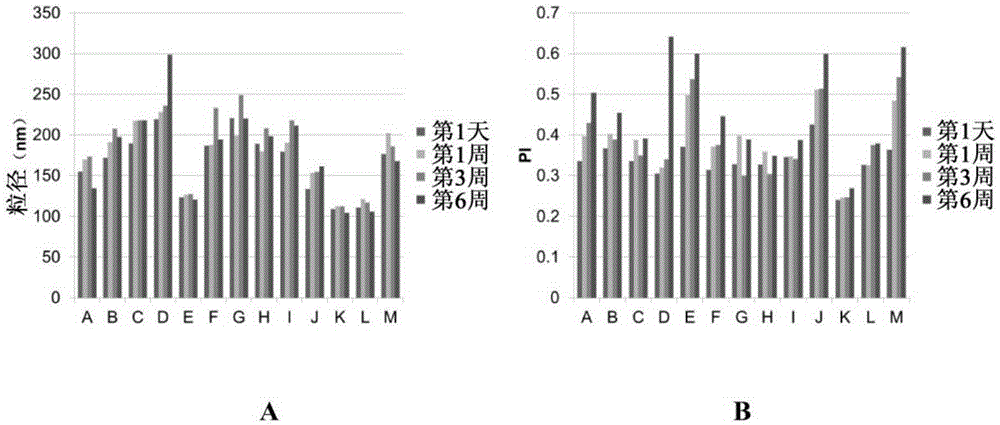
[0200] Embodiment 2: the selection of liquid lipid
[0201] The properties of lipid nanodispersions are compared to select better liquid lipids for final formulations. Using the method described above, lipid nanodispersions were prepared using MCT (lipid nanodispersion N) or castor oil (lipid nanodispersion O) with the following formulation:
[0202]
[0203] result:
[0204] Table 6 Properties of lipid nanodispersion N and O
[0205]
[0206] The data in Table 6 shows that the particle size of the formulation prepared with castor oil was much larger than that of the formulation prepared with MCT. Lipid nanodispersion O is therefore less desirable than lipid nanodispersion N. The |zeta potential| of lipid nanodispersion N is much larger than the |zeta potential| of lipid nanodispersion O. Therefore, lipid nanodispersion N generally has better stability compared to lipid nanodispersion O. like Figure 4 As shown, Lipid Nanodispersion N (left bottle) has a mor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com