Hydraulic urethral occlusive device
A closure device, hydraulic fluid technology, used in incontinence prevention devices, devices for human tubular structures, medical science, etc., can solve problems such as difficulty in teaching patients to identify and operate locking valves
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
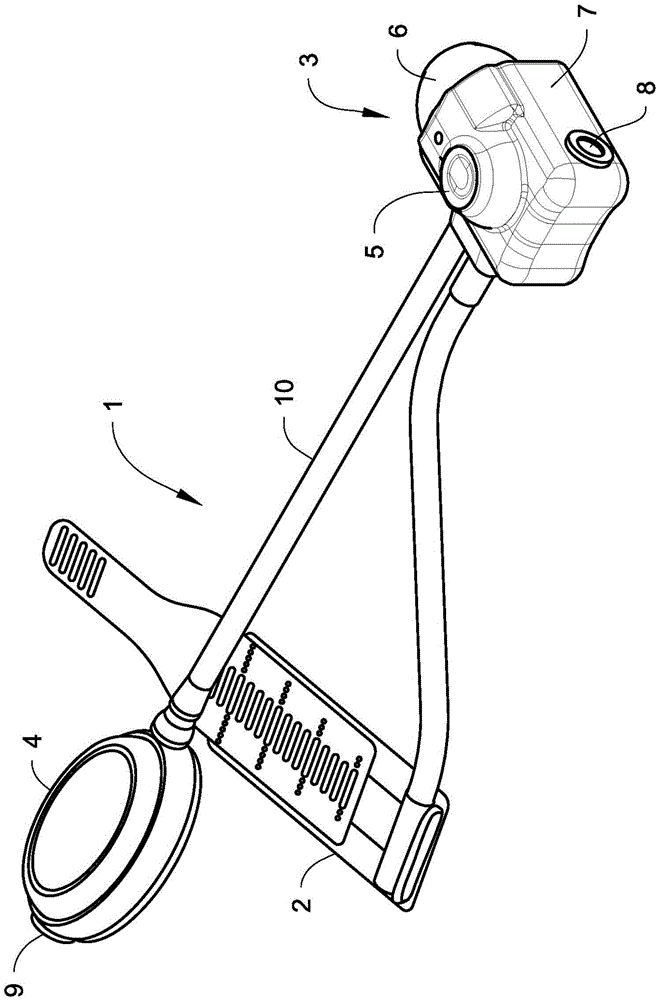
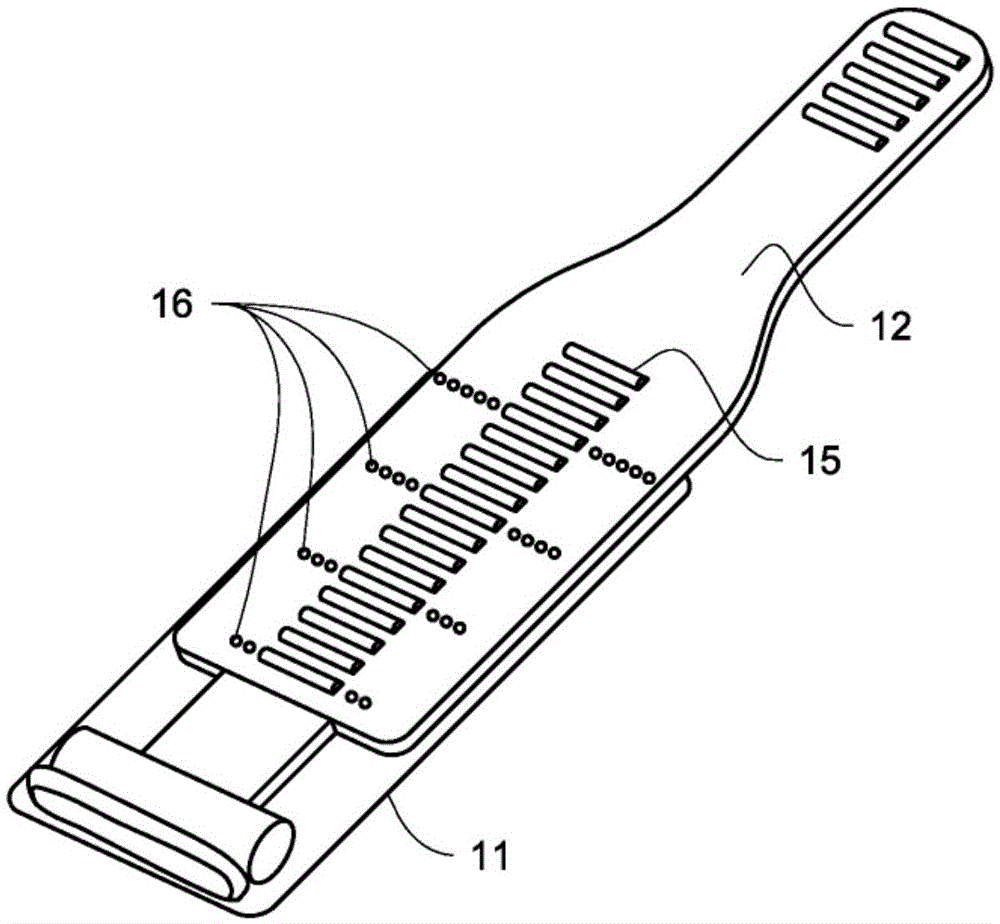
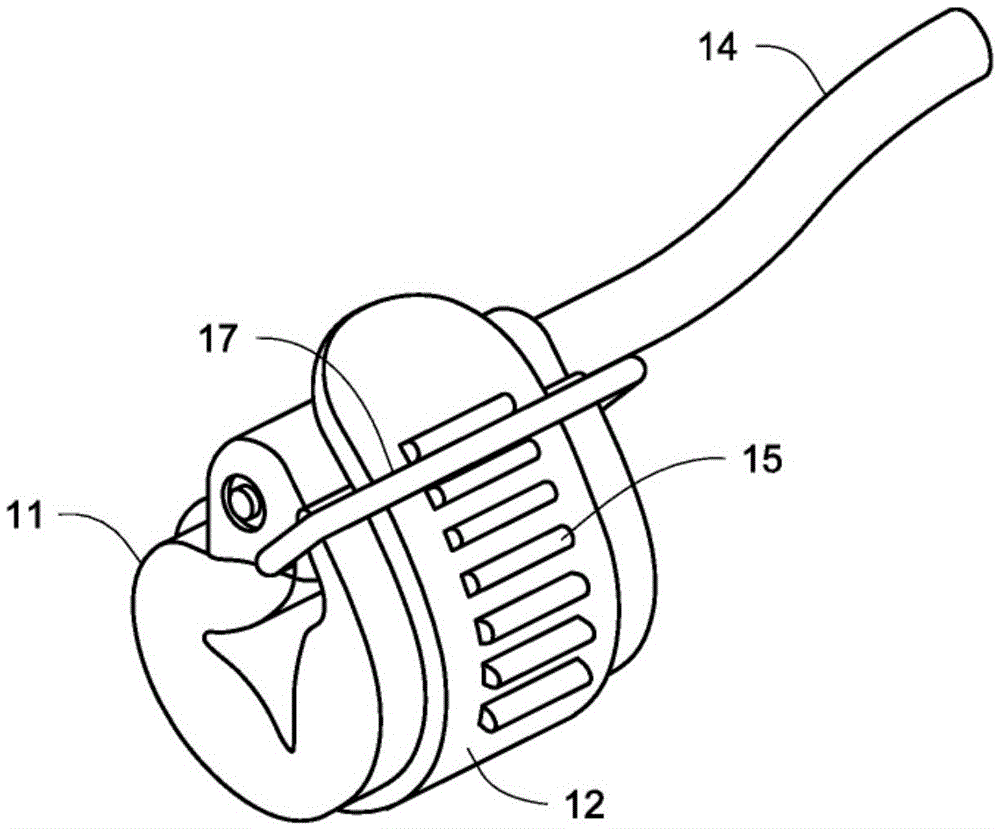
[0026] figure 1shows a hydraulic urethral closure device (HUOD) 1 implanted in a male body as shown. Hydraulic urethral closure devices are one-piece devices that are implanted through a perineal or penoscrotal incision. An inflatable hydraulic closure cuff 2 encircles the urethra and is permanently connected via a flexible tube to a control mechanism 3 implanted in the scrotum. The pressure compensator 4 is also permanently connected to the control mechanism 3 via a flexible catheter 10 so as to be able to be placed in the subcutaneous tissue of the abdomen, thigh or alternatively in the prevesical space. The closure cuff 2 is implanted in the unexpanded released state and left in place for approximately 6 weeks postoperatively to allow for healing and resolution of pain and edema. After this release time, the urologist activates the device by pressing the activation button 5 with the intact scrotal skin. In doing so, the closure cuff 2 is inflated to apply a preset 60-80 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com