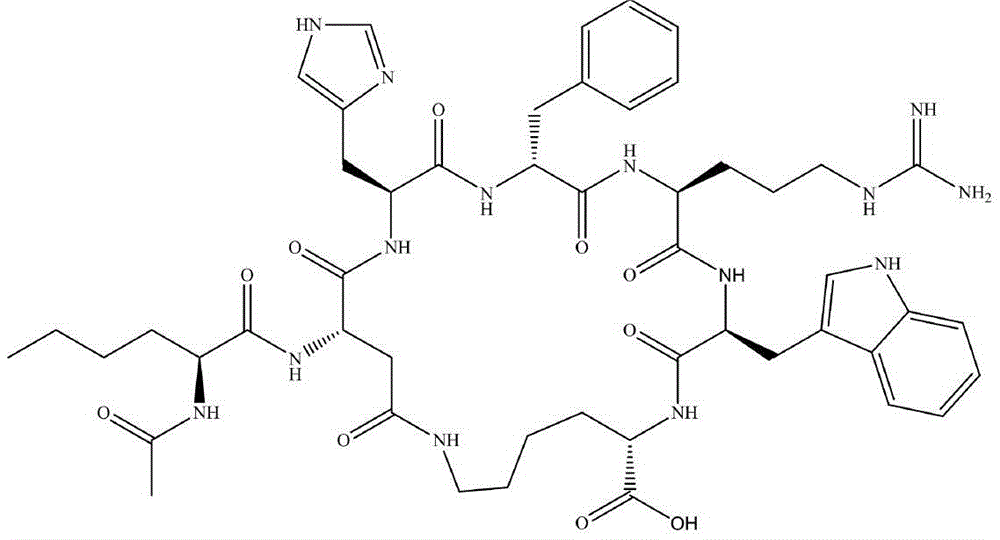
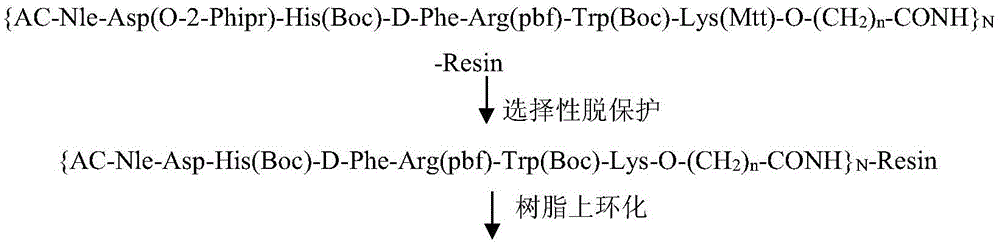
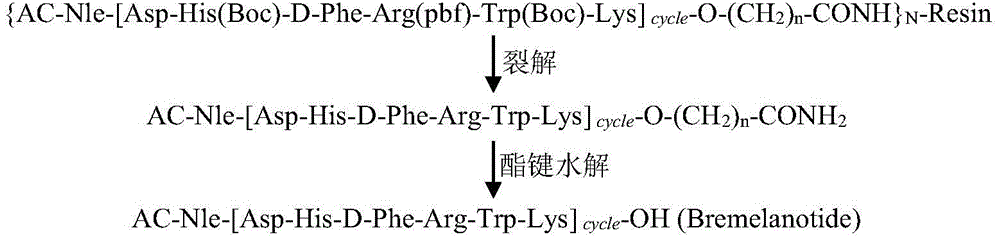
Solid-phase synthesis method of bremelanotide
A technology of bremelanotide and solid-phase synthesis, which is applied to the preparation method of peptides, chemical instruments and methods, and peptides, and can solve problems such as difficult ring formation and large steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: HO-(CH 2 ) 7 - Preparation of CONH-RinkAmideResin
[0071] Accurately weigh 100g of RinkAmideResin with a substitution degree of 0.52mmol / g and place it in a solid-phase synthesis kettle, wash twice with DMF, 500ml / time, 3min / time, after washing, add 500mlDCM to swell the resin for 30min.
[0072] RinkAmideResin was deprotected with 20% piperidine / DMF, 400ml / time, the first reaction was 5min, and the second reaction was 15min. After deprotection twice, the resin was washed six times with DMF, 500ml / time, 3min / time. Take a small sample of the resin and detect it with ninhydrin reagent, the resin is dark blue.
[0073] Weigh HO-(CH 2 ) 7 -COOH24.9g (156mmol, 3eq), HOBt23.2g (171.6mmol, 3.3eq) were placed in a dissolving bottle, dissolved in 200ml of DMF, pre-cooled at 0-5°C for 10min, and activated by adding DIC27.5ml (171.6mmol, 3.3eq) 5min, put into the resin after the activation is completed, stir and react at 25°C for 2h. After 2 hours, the resin was...
Embodiment 2
[0074] Example 2: HO-(CH 2 ) 15 - Preparation of CONH-RinkAmideResin
[0075] Accurately weigh 100g of RinkAmideResin with a substitution degree of 0.52mmol / g and place it in a solid-phase synthesis kettle, wash twice with DMF, 500ml / time, 3min / time, after washing, add 500mlDCM to swell the resin for 30min.
[0076] RinkAmideResin was deprotected with 20% piperidine / DMF, 400ml / time, the first reaction was 5min, and the second reaction was 15min. After deprotection twice, the resin was washed six times with DMF, 500ml / time, 3min / time. Take a small sample of the resin and detect it with ninhydrin reagent, the resin is dark blue.
[0077] Weigh HO-(CH 2 ) 15 -COOH42.4g (156mmol, 3eq), HOBt23.2g (171.6mmol, 3.3eq) was placed in a dissolving bottle, dissolved in 200ml of DMF, pre-cooled at 0-5°C for 10min, and activated by adding DIC27.5ml (171.6mmol, 3.3eq) 5min, put into the resin after the activation is completed, stir and react at 25°C for 2h. After 2 hours, the resin wa...
Embodiment 3
[0078]Example 3: Fmoc-Lys(Mtt)-O-(CH 2 ) 7 - Preparation of CONH-RinkAmideResin
[0079] Accurately weigh Fmoc-Lys(Mtt)-OH97.344g (156mmol, 3eq), HOBt23.2g (171.6mmol, 3.3eq) into a dissolving bottle, dissolve with 300ml DMF, pre-cool at 0-5°C for 10min, add DIC27. 5ml (171.6mmol, 3.3eq) activation 5min, drop into the synthetic resin HO-(CH of embodiment 1 after activation completes 2 ) 7 -CONH-RinkAmideResin, after stirring for 10 minutes, add DMAP 1.9g (15.6mmol, 0.3eq), and stir for 5 hours at 25°C. After 5h, the resin was washed and dried in a vacuum drying phase (<-0.1Mpa, 8h). A sample was taken to measure the degree of substitution, and the measured value was 0.33mmol / g.
[0080] Weigh 90g of the above dry resin (substitution degree is 0.33mmol / g, 30mmol) and place it in a solid-phase synthesis kettle, wash twice with DMF, 500ml / time, 3min / time, add 500mlDCM to swell the resin for 30min after washing.
[0081] Measure 45ml (450mmol, 15eq) of acetic anhydride and 3...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap