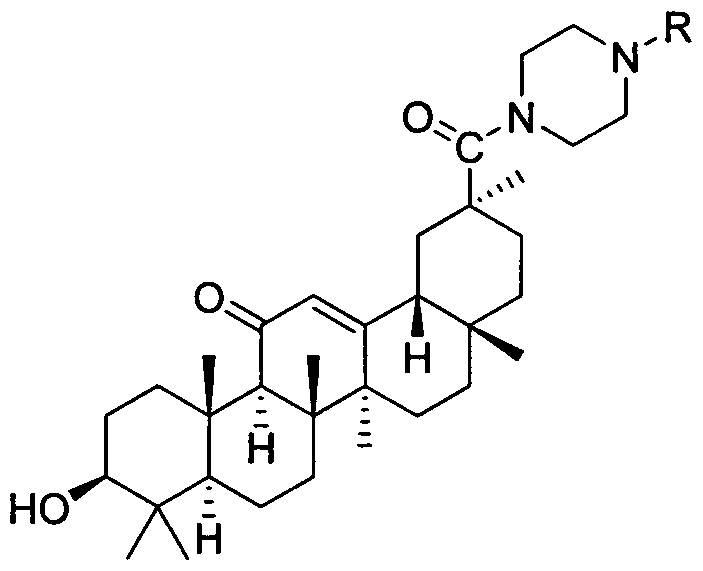
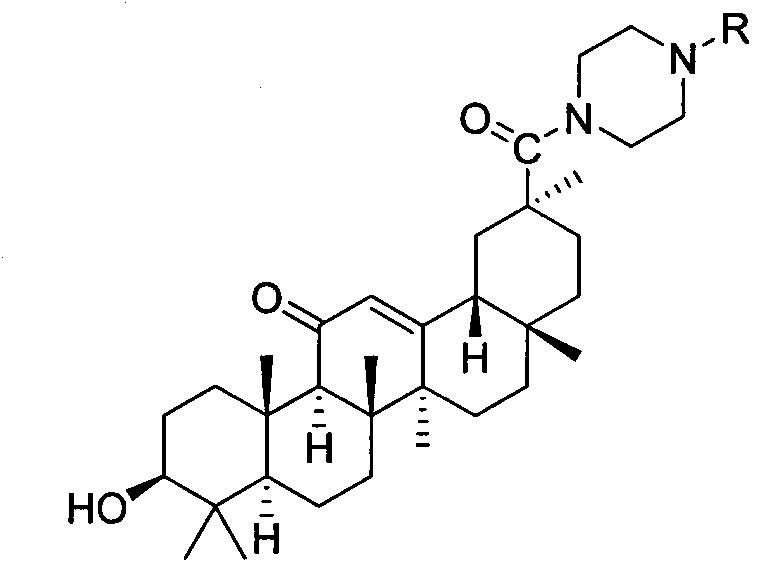
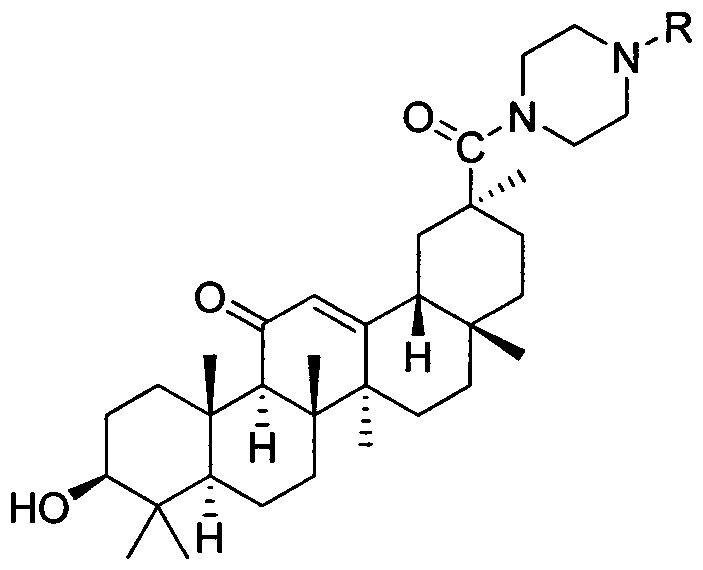
Piperazine-framework-containing glycyrrhetinic acid derivatives, and preparation method and application thereof
A technique for glycyrrhetinic acid and derivatives, applied in the field of glycyrrhetinic acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 3βhydroxy-30-(4-phenyl-1-piperazinyl)-olean-12-ene-11,30-dione (3a)
[0021]
[0022] At room temperature, 20 mL of dichloromethane solution was added to the reaction vessel, followed by adding 1.2 mmol each of DCC and HOBT, stirring at room temperature for 30 min, then adding glycyrrhetinic acid (1 mmol) and stirring at room temperature for 20 min. Then phenylpiperazine (1.2mmol) and triethylamine (3mmol) were stirred at room temperature for 24h, and the reaction was tracked by TLC. After sufficient reaction, compound 3a was obtained. Yield: 83%.M.p.282-284℃. 1 HNMR (400MHz, DMSO-d 6 ): δ (ppm) 7.20-7.27 (m, 2H, Ar-7', 9'), 6.94 (d, J=7.92Hz, 2H, Ar-6', 10'), 6.81 (t, J=7.24 Hz, 1H, Ar-8'), 5.48(s, 1H, CH-12), 4.32(d, J=5.16Hz, 1H, -OH), 3.70(s, 4H, CH 2 -1', CH 2 -4'), 3.10(t, J=4.60Hz, 4H, CH 2 -2', CH 2 -3'), 2.98-3.05(m, 1H, CH-3), 2.57(d, J=13.32Hz, 1H, CH-18), 2.33(s, 1H, CH-9), 2.26-1.36(m , 16H, CH 2 -2,CH 2 -6,CH 2 -7,CH ...
Embodiment 2
[0023] Example 2: Preparation of 3βhydroxy-30-(4-(2,4-dimethylphenyl)-1-piperazinyl)-olean-12-ene-11,30-dione (3b)
[0024]
[0025] The preparation method refers to Example 1. Yield: 79%. M.p.175-177℃. 1 HNMR (400MHz, DMSO-d 6 ): δ (ppm) 6.88-7.00 (m, 3H, Ar-6'7', 9'), 5.52 (s, 1H, CH-12), 4.32 (d, J=5.16Hz, 1H, -OH) , 3.68(s, 4H, CH 2 -1', CH 2 -4'), 2.99-3.05(m, 1H, CH-3), 2.75(s, 4H, CH 2 -2', CH 2 -3'), 2.57(d, J=13.36Hz, 1H, CH-18), 2.33(s, 1H, CH-9), 2.22(d, J=7.04Hz, 6H, Me-8', Me- 10'), 2.17-1.37(m, 16H, CH 2 -2,CH 2 -6,CH 2 -7,CH 2 -15,CH 2 -16,CH 2 -19, CH 2 -21,CH 2 -22), 1.35(s, 3H, Me-27), 1.19(s, 3H, Me-29), 1.03(d, J=1.64Hz, 6H, Me-25, Me-26), 0.97(d, J=14.32Hz, 2H, CH 2 -1), 0.91(s, 3H, Me-23), 0.75(s, 3H, Me-24), 0.71(s, 1H, CH-5), 0.69(s, 3H, Me-28).
Embodiment 3
[0026] Example 3: Preparation of 3βhydroxy-30-(4-(4-nitrophenyl)-1-piperazinyl)-olean-12-ene-11,30-dione (3c)
[0027]
[0028] The preparation method refers to Example 1. Yield: 74%. M.p.252-254℃. 1 HNMR (400MHz, DMSO-d 6 ): δ (ppm) 8.09 (d, J=9.44Hz, 2H, Ar-7', 9'), 7.00 (d, J=9.52Hz, 2H, Ar-6', 10'), 5.50(s, 1H, CH-12), 4.32 (d, J=5.16Hz, 1H, -OH), 3.72 (s, 4H, CH 2 -1', CH 2 -4'), 3.48(d, J=4.56Hz, 4H, CH 2 -2', CH 2 -3'), 2.99-3.05(m, 1H, CH-3), 2.57(d, J=13.36Hz, 1H, CH-18), 2.33(s, 1H, CH-9), 2.24-1.36(m , 16H, CH 2 -2,CH 2 -6,CH 2 -7,CH 2 -15,CH 2 -16,CH 2 -19, CH 2 -21,CH 2 -22), 1.35(s, 3H, Me-27), 1.19(s, 3H, Me-29), 1.03(s, 6H, Me-25, Me-26), 0.97(d, J=13.64Hz, 2H, CH 2 -1), 0.91(s, 3H, Me-23), 0.74(s, 3H, Me-24), 0.71(s, 1H, CH-5), 0.69(s, 3H, Me-28).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap