Levomilnacipran hydrochloride sustained release capsule and preparation method thereof
A technology of milnacipran hydrochloride and sustained-release capsules is applied in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc. Well-tolerated, easy-to-use results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
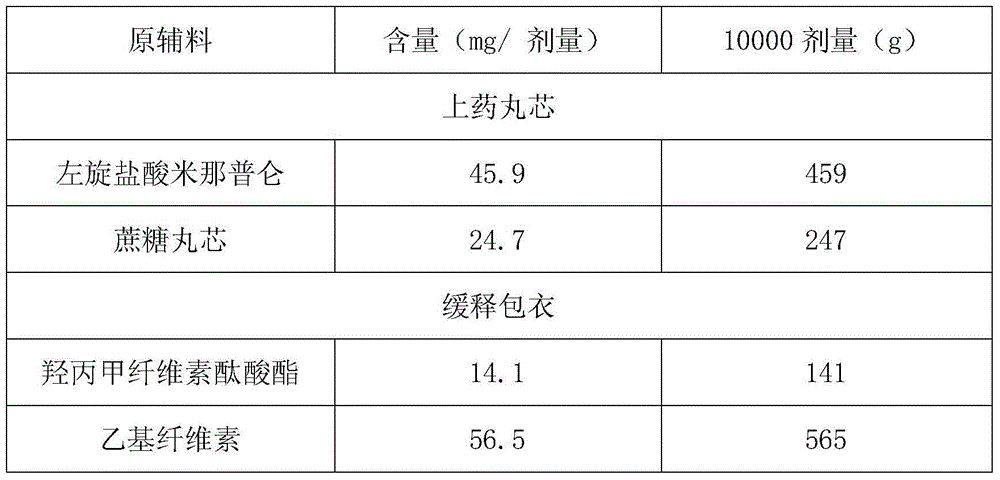
[0025]
[0026] 459 grams of L-milnacipran hydrochloride are uniformly dispersed in 1836 grams of isopropanol, and the resulting drug-on-dose suspension is sprayed on 247 grams of 30-35 mesh sucrose cores using a fluidized bed GPCG2, and dried to obtain drug-containing Small Maru.
[0027] 141 grams of hypromellose phthalate and 565 grams of ethyl cellulose are dissolved in a mixed solvent of acetone and isopropanol to make a coating solution; use the fluidized bed GPCG2 at a temperature of 26°C The coating liquid is spray-coated on the drug-containing pellets of L-milnacipran hydrochloride, and the weight of the coating is increased by 50%, so as to obtain the coated pellets.
Embodiment 2
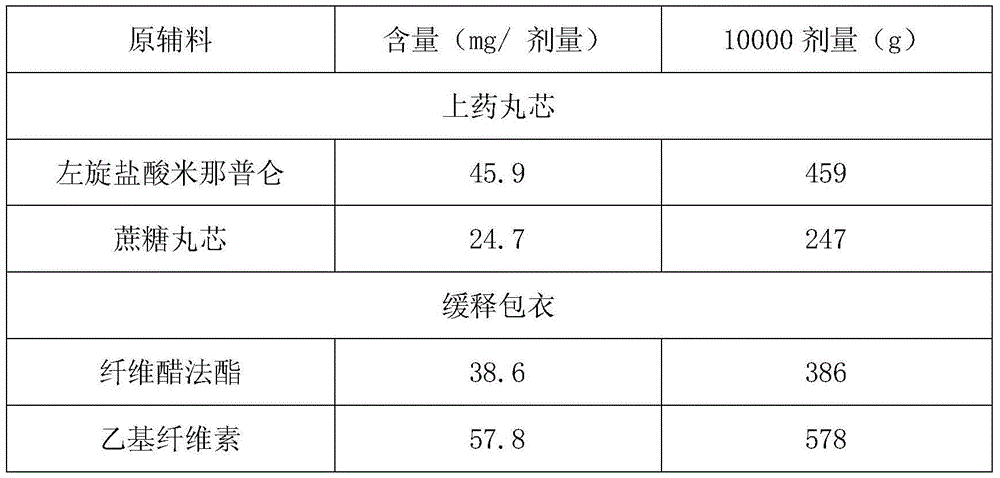
[0029]
[0030] 459 grams of L-milnacipran hydrochloride are uniformly dispersed in 1836 grams of isopropanol, and the resulting drug-on-dose suspension is sprayed on 247 grams of 30-35 mesh sucrose cores using a fluidized bed GPCG2, and dried to obtain drug-containing Small Maru.
[0031] Dissolve 578 grams of ethyl cellulose and 386 grams of cellulose acetate in a mixed solvent of acetone and isopropanol to make a coating solution; use the fluidized bed GPCG2 at a temperature of 26 ° C to spray the coating solution Coating on the drug-containing pellets of L-milnacipran hydrochloride, the coating weight increased by 57.7%, to obtain the coated pellets. The coated pellets were filled into capsules, and the release of the preparation at each set time point at 100 rpm in the USP device 1 (small basket) at 37° C. in pH6.8 buffer was as follows: 17% in 2 hours, released in 4 hours 41%, 62% released in 8 hours, 87% released in 12 hours.
Embodiment 3
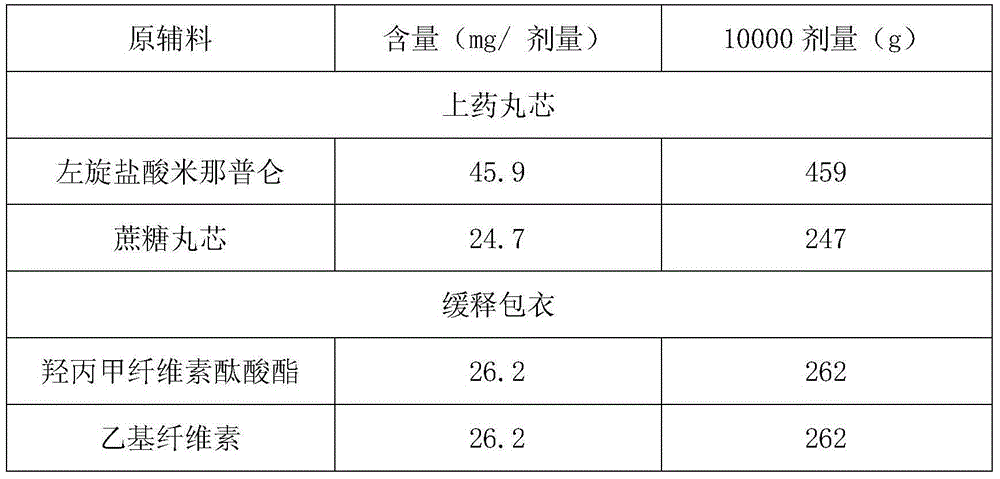
[0033]
[0034] 459 grams of L-milnacipran hydrochloride are uniformly dispersed in 1836 grams of isopropanol, and the resulting drug-on-dose suspension is sprayed on 247 grams of 30-35 mesh sucrose cores using a fluidized bed GPCG2, and dried to obtain drug-containing Small Maru.
[0035] Dissolve 262 grams of ethyl cellulose and 262 grams of hypromellose phthalate in a mixed solvent of acetone and ethanol to make a coating solution; use the fluidized bed GPCG2 at a temperature of 26 ° C to use the coating solution Spray coating on the drug-containing pellets of L-milnacipran hydrochloride, the coating weight increased by 31.4%, to obtain the coated pellets.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap